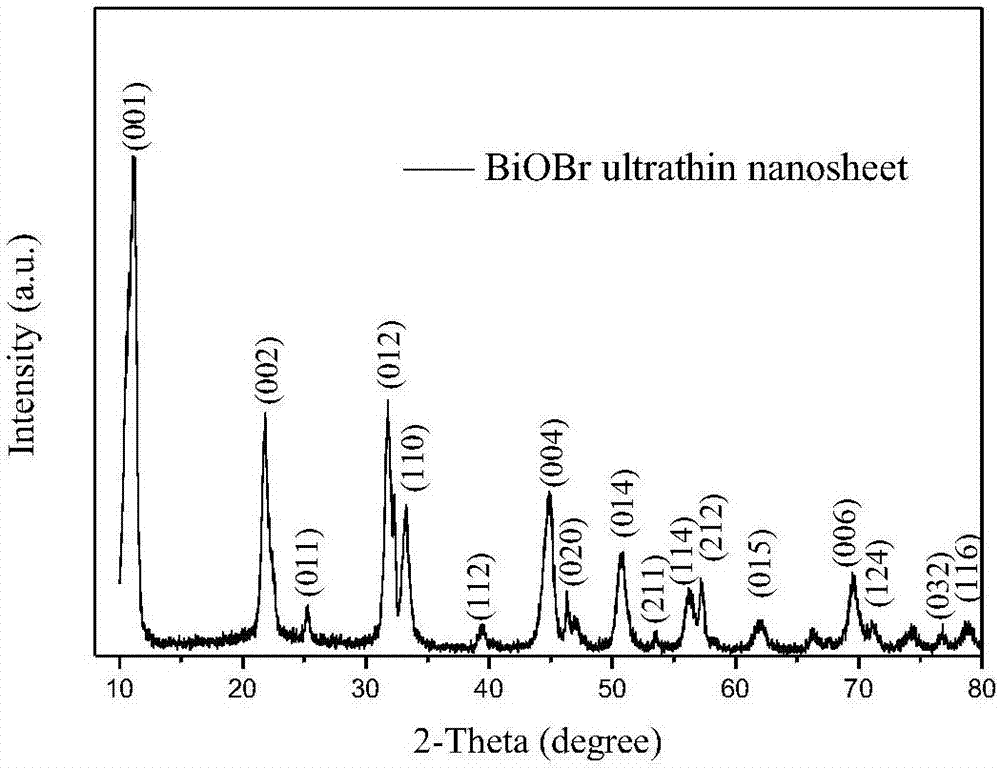
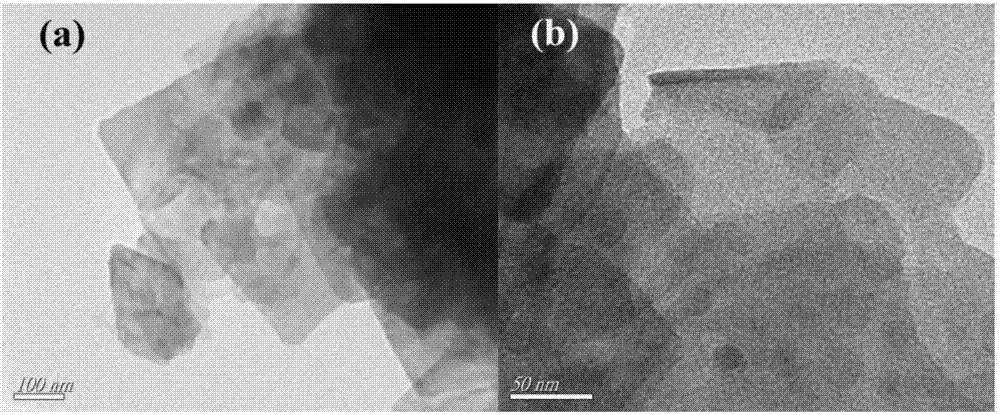
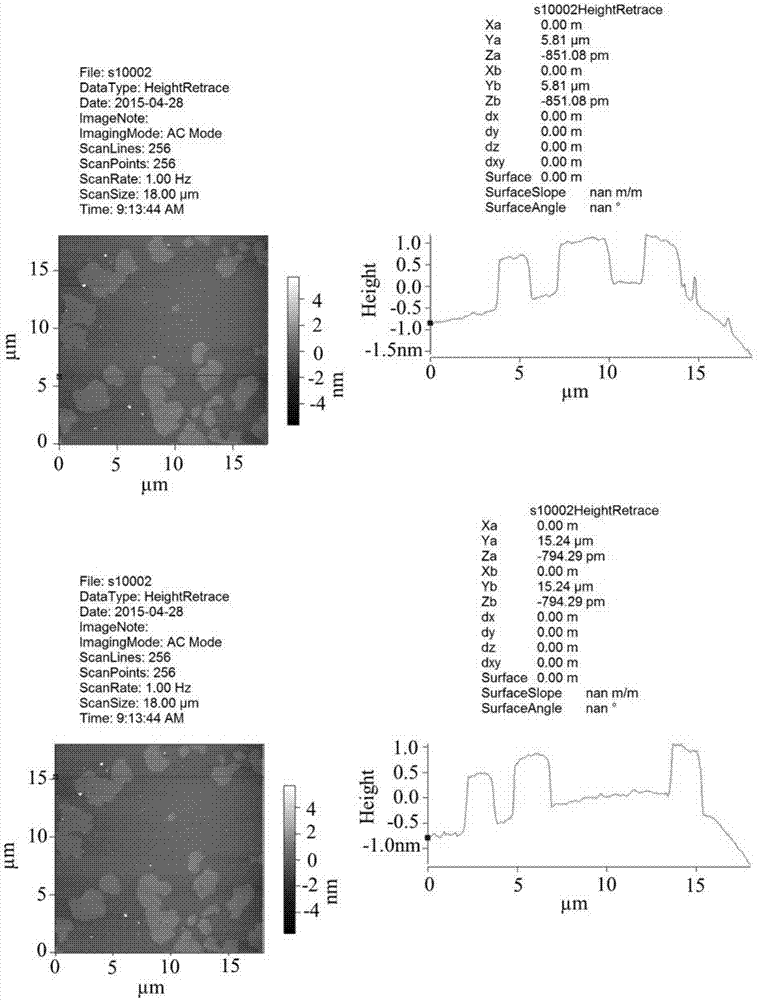
Method of synthesizing bismuth oxybromide ultrathin nanosheet photocatalyst at room temperature
A technology of bismuth oxybromide and photocatalyst, applied in physical/chemical process catalysts, chemical instruments and methods, inorganic chemistry, etc., to achieve the effect of short synthesis time, thin thickness and efficient degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Taking 1-butyl-3-methylimidazole bromide as the step of bromine source to prepare photocatalyst BiOBr ultra-thin nanosheets:
[0030] Using bismuth nitrate as raw material, A solution was formulated in a mixed solvent of 10mL water and acetic acid (volume ratio: 9:1), wherein the content of bismuth was 0.01-0.2mol / L, and in another container, bromide ions were used The liquid is the raw material, and it is prepared in 10mL of ethanol solvent to prepare solution B, in which the content of bromine is 0.01-0.6mol / L. Under the condition that solution A is stirred, add solution B dropwise to solution A, and mix the obtained solution The solution was stirred at room temperature for 5-120 minutes, the product was centrifuged, washed several times with deionized water and absolute ethanol, and dried at 50° C. for 5-24 hours to obtain a solid powder.
Embodiment 2
[0032] Adopt bismuth nitrate as raw material, be made into A solution in the mixed solvent of 10mL water and acetic acid (volume ratio is 9:1), wherein the content of bismuth is 0.01mol / L, in another container, adopt bromide ionic liquid as The raw material is formulated into solution B in 10mL of ethanol solvent, wherein the content of bromine is 0.05mol / L. Under the condition of stirring solution A, add solution B dropwise to solution A, and continue to stir the resulting mixed solution at room temperature After 5 minutes, the product was centrifuged, washed several times with deionized water and absolute ethanol, and dried at 50° C. for 5 hours to obtain a solid powder.
Embodiment 3
[0034] Adopt bismuth nitrate as raw material, be made into A solution in the mixed solvent of 10mL water and acetic acid (volume ratio is 9:1), wherein the content of bismuth is 0.1mol / L, in another container, adopt bromide ionic liquid as The raw material is formulated into solution B in 10mL ethanol solvent, wherein the content of bromine is 0.3mol / L. Under the condition of stirring solution A, add solution B dropwise to solution A, and continue to stir the resulting mixed solution at room temperature After 60 minutes, the product was centrifuged, washed several times with deionized water and absolute ethanol, and dried at 50°C for 10 hours to obtain a solid powder.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com