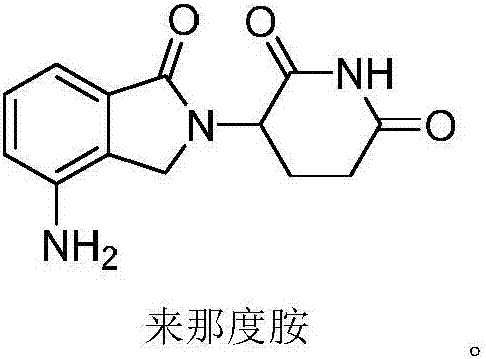
Preparation method of lenalidomide for treating multiple myeloma
A technology of lenalidomide and amino, which is applied in the field of drug synthesis, can solve the problems of low product yield and complicated steps, and achieve the effect of simple and convenient preparation and suitable for industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] A preparation method for lenalidomide for treating multiple myeloma, the preparation method comprising the following steps:
[0032] 1) 19.5g (100mmol) of methyl 2-methyl-3-nitrobenzoate, 14.1g (110mmol) of 3-amino-2,6-piperidinedione, 58.1g (500mmol) of tetramethylethylenediamine ), 48.8g (160mmol) of zinc bromide were added to the reaction vessel, 250ml of 1,4-dioxane was added and the temperature was raised to 90°C for a mixed reaction for 10 to 16 hours. After the reaction, cooled to room temperature and concentrated under reduced pressure , washed with water, and recrystallized (petroleum ether:dichloromethane=10:1) to obtain a white solid 3-(7-nitro-3-oxo-1H-isoindol-1-yl)piperidine-2,6- Diketone 26.1g, yield 90.1%, HPLC purity 99.32%; 1 HNMR (300MHz, DMSO-d 6 )δ:11.04(s,1H),8.47(d,1H),8.19(d,1H),7.86(t,1H),5.20-5.17(m,1H),4.94-4.82(m,2H),2.95 -2.88(m,1H),2.63-2.50(m,2H),2.05-2.01(m,1H).
[0033] 2) Add 10 g of 3-(7-amino-3-oxo-1H-isoindol-1-yl)piperidine-2,6-...
Embodiment 2
[0035] A preparation method for lenalidomide for treating multiple myeloma, the preparation method comprising the following steps:
[0036] 1) 19.5g (100mmol) of methyl 2-methyl-3-nitrobenzoate, 14.1g (110mmol) of 3-amino-2,6-piperidinedione, 46.5g (400mmol) of tetramethylethylenediamine ), zinc bromide 54.9g (180mmol) joins in the reaction container, adds 250ml toluene and heats up to 95 ℃ and carries out mixed reaction 10~16 hours, after reaction finishes, cools to room temperature, concentrates under reduced pressure, washes with water, recrystallizes (petroleum Ether: dichloromethane=10:1) to obtain 25.9 g of 3-(7-nitro-3-oxo-1H-isoindol-1-yl)piperidine-2,6-dione with a yield of 89.4 %, HPLC purity 99.62%;
[0037] 2) Add 10 g of 3-(7-amino-3-oxo-1H-isoindol-1-yl)piperidine-2,6-dione and 0.5 g of 5% palladium carbon to a high pressure with 18 ml of ethanol In the kettle, feed hydrogen (the pressure of feeding hydrogen is 0.5 MPa), and perform a reduction reaction at 50 °...
Embodiment 3
[0039] A preparation method of lenalidomide for the treatment of multiple myeloma, the preparation method comprising the following steps:
[0040] 1) 19.5 g (100 mmol) of methyl 2-methyl-3-nitrobenzoate, 15.4 g (120 mmol) of 3-amino-2,6-piperidinedione, 58.1 g (500 mmol) of tetramethylethylenediamine ), zinc bromide 45.8g (150mmol) are joined in the reaction vessel, add 250ml 1,4-dioxane and be warming up to 95 ℃ to carry out mixed reaction 10~16 hours, after the reaction finishes, be cooled to room temperature, concentrated under reduced pressure , washed with water, and recrystallized (petroleum ether:dichloromethane=10:1) to obtain 3-(7-nitro-3-oxo-1H-isoindol-1-yl)piperidine-2,6-dione 26.2g, yield 90.7%, HPLC purity 99.51%;
[0041] 2) 10 g of 3-(7-amino-3-oxo-1H-isoindol-1-yl) piperidine-2,6-dione and 0.3 g of 5% palladium on carbon were added to a high pressure containing 15 ml of ethanol. In the kettle, feed hydrogen (the pressure of feeding hydrogen is 0.35MPa), at 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com