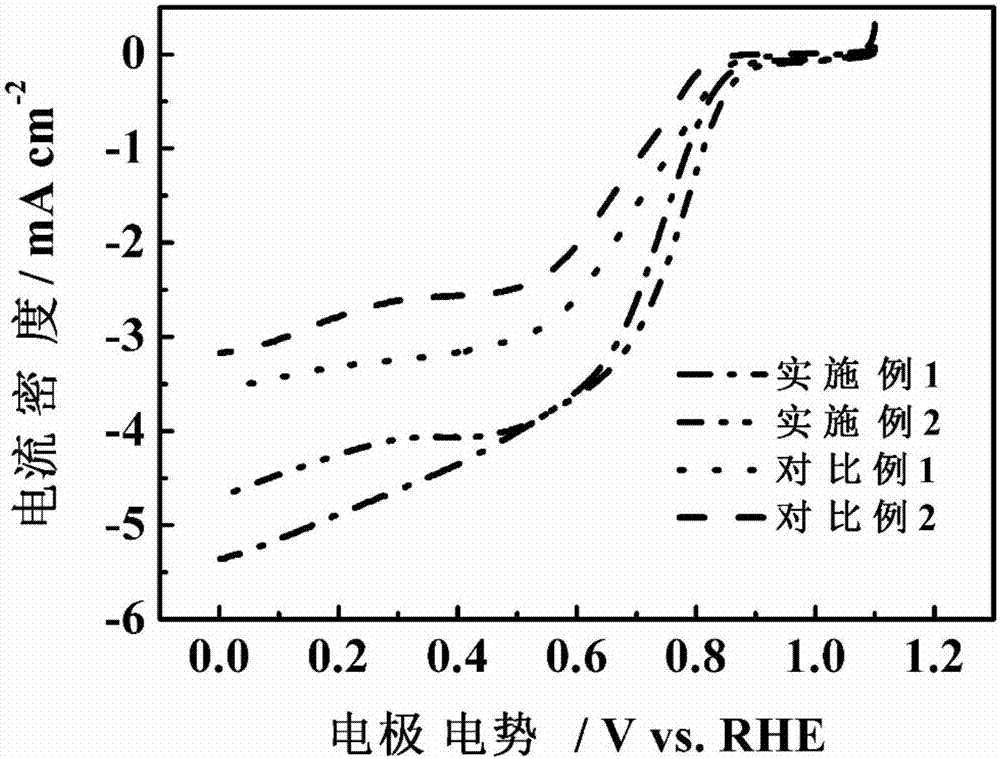
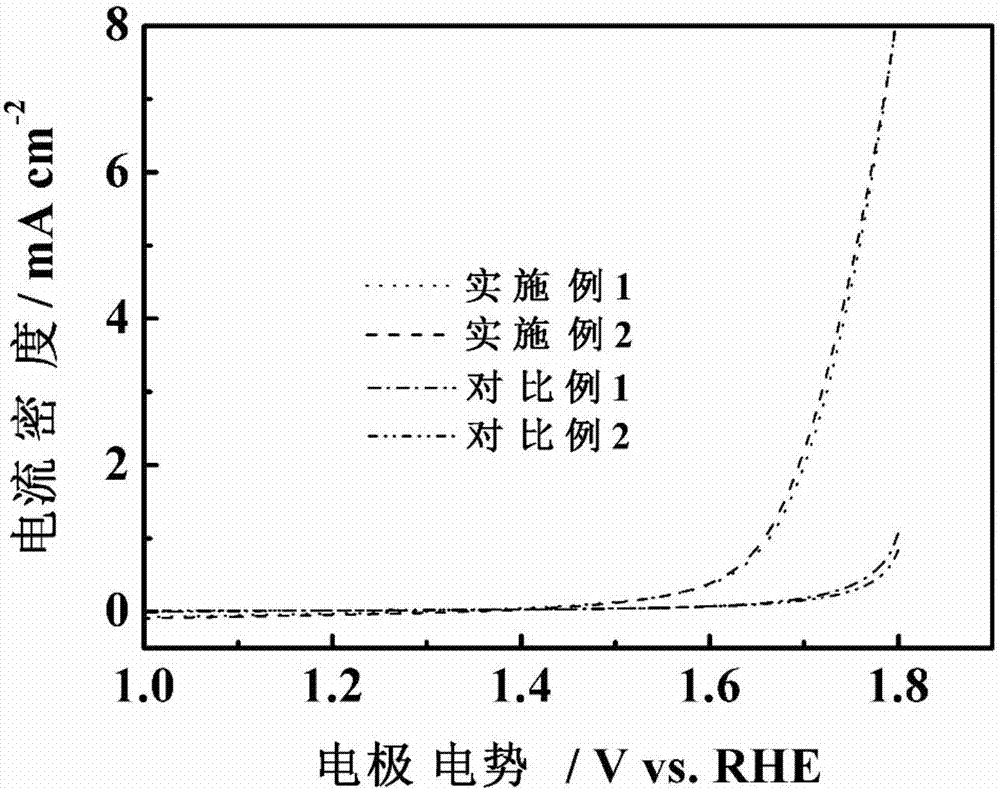
Nitrogen and phosphor-doped carbon-based nonmetallic oxygen reduction/separation double-effect catalyst and preparation method thereof
A co-doping, non-metallic technology, applied in structural parts, electrical components, battery electrodes, etc., can solve the problems of high cost, difficulty in large-scale promotion, poor stability, and low catalytic performance of secondary metal-air battery catalysts, and achieve high-efficiency oxygen Effects of precipitation reaction catalytic performance, mild synthesis conditions, and good double-effect electrocatalytic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Weigh 0.238g (0.907mmol) of triphenylphosphine, 0.180g (1.428mmol) of melamine, and 0.060g of graphene oxide into 20ml of tetrahydrofuran, ultrasonically mix, then transfer to a 50ml reactor, heat up to 110°C, and react 24 hours.
[0030] The resulting product was washed with deionized water and ethanol for several times, and dried in an oven at 60°C for 12 hours. Carbon-based materials containing nitrogen and phosphorus are obtained. The synthesized nitrogen- and phosphorus-containing carbon-based materials were moved into a high-temperature furnace for calcination, with a flow rate of 100mL min -1 Argon gas, the high temperature furnace is heated up to 350°C at a rate of 5-10°C / min, held for 2 hours, then heated to 900°C, and then cooled naturally after holding for 2 hours to form a nitrogen-phosphorus co-doped carbon-based double-effect catalyst.
Embodiment 2
[0032] Weigh 0.190g (0.546mmol) of hexachlorotrimeric phosphazene, 0.200g (1.587mmol) of melamine, and 0.060g of carbon nanotubes into 20ml of tetrahydrofuran, mix well by ultrasonic, then transfer to a 50ml reaction kettle, and heat up to 120°C , reacted for 24 hours.
[0033] The resulting product was washed with deionized water and ethanol for several times, and dried in an oven at 70°C for 12 hours. Carbon-based materials containing nitrogen and phosphorus are obtained. The synthesized carbon-based materials containing nitrogen and phosphorus are moved into a high-temperature furnace for calcination, with a flow rate of 100mL min -1 Argon gas, the high temperature furnace is heated up to 350°C at a rate of 5-10°C / min, held for 3 hours, then heated to 950°C, and then cooled naturally after holding for 2 hours to form a nitrogen-phosphorus co-doped carbon-based double-effect catalyst.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com