Cell composition and preparation method and application thereof
A composition and cell technology, applied in the directions of drug combinations, pharmaceutical formulations, and medical preparations containing active ingredients, can solve the problems of inability to cure Crohn's disease, imbalance of the immune system, and inability to effectively prevent the disease. Disease progression, maintenance of biological activity, remarkable effect of treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 1. Isolation and extraction of intestinal stem cells, reference: Ye Zhangqun, Liu Guanlin, Chen Zhiqiang. Construction of mouse intestinal stem cell population. "Journal of Huazhong University of Science and Technology (Medical Edition)" [J], 2006.
[0029] Take intestinal stem cells from passage P2 to passage P5, suck out the medium, add 0.25% trypsin for routine digestion, and then make the cell density 1×10 5 cells / mL cell suspension, then add 5 μL anti-Lgr5 monoclonal antibody to 500 μL cell suspension, then incubate at room temperature in the dark for 20 min, and set up a blank isotype control at the same time; after incubation, centrifuge at 1500 r / min for 5 min, discard The supernatant was washed twice with PBS containing 10% FBS, resuspended with 500 μL of PBS, and tested on the machine.
[0030] The surface antigen detection results of intestinal stem cells are shown in Table 1 below:
[0031] Table 1
[0032]
Lrg5 +
first detection
...
Embodiment 2
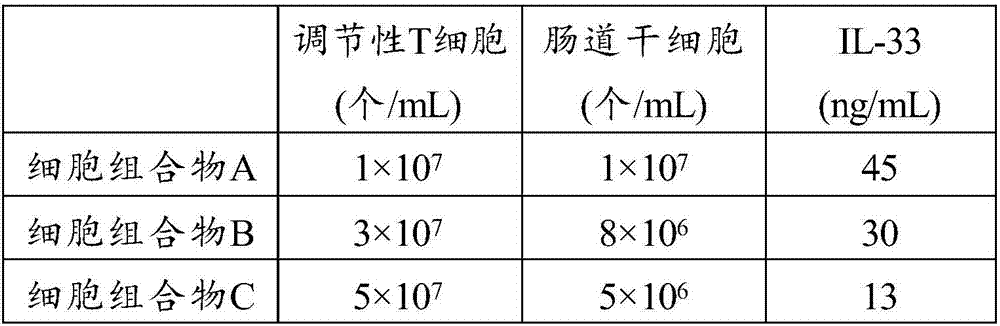
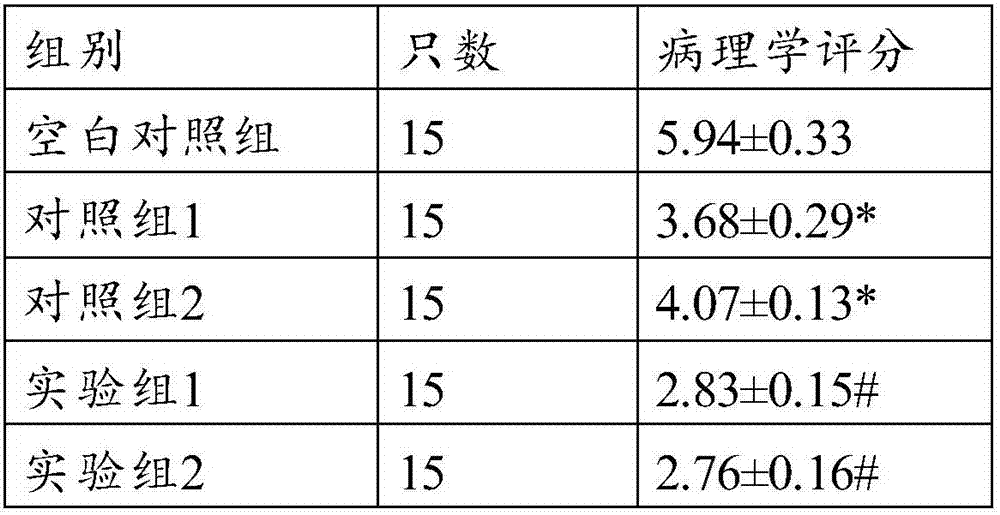
[0042] 1, randomly select the mice of 90 SPF grades of similar body weight, divide mice into 6 groups, every group of 15 (male and female); then, each mouse is carried out trinitrobenzenesulfonic acid (2,4, 6-Trinitrobenzenesulfonic Acid, TNBS) enema to manufacture the mouse CD model; then, each model mouse was injected intravenously along the tail according to Table 4, injected once a week, and injected continuously for 4 times, and after 1 month of such conventional feeding All the mice were sacrificed by dislocation, and all indexes were detected.
[0043] References for Crohn's mouse modeling: Liu Shun, Sun Keming, Yang Houlai. Study on the therapeutic effect and mechanism of rapamycin on Crohn's disease mouse model. "Chinese Journal of Endemic Diseases" [J]. 2016 .
[0044] Table 4 Preparations for Injection
[0045] group
injection
Blank control group
normal saline
Control group 1
Infliximab
Control group 2
immunosuppressa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com