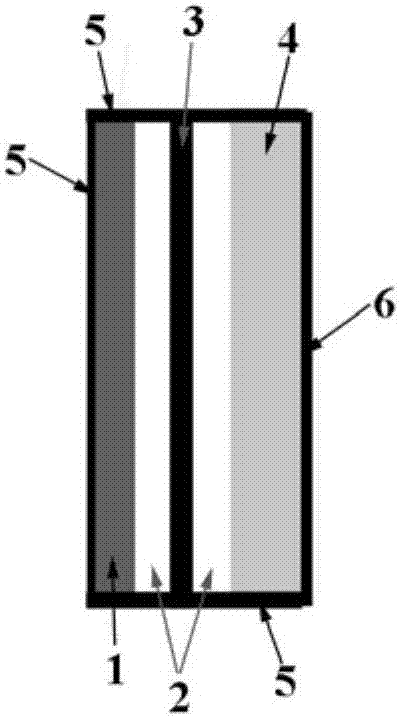
Metal-CO2 battery
A carbon dioxide, battery technology, applied in battery electrodes, circuits, electrical components, etc., can solve the problems of electrode passivation, high decomposition overpotential, and unsatisfactory cycle life.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
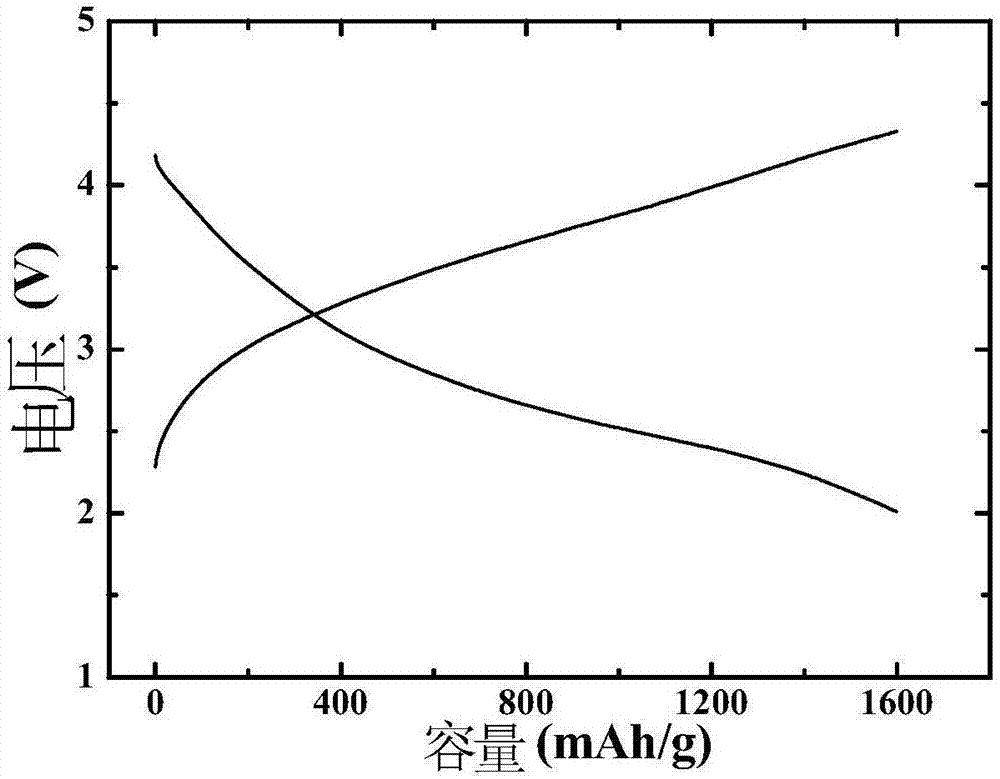
Embodiment 1
[0059] After the commercial carbon cloth was hydrothermally reacted in 69wt% concentrated nitric acid at 90°C for 2 hours, it was first washed with deionized water, then ultrasonically cleaned with acetone, alcohol, and deionized water for 15 minutes, and then heated in air at 60°C. Dry for 8h; KMnO 4 Dissolve in deionized water and stir well to obtain K + A solution with a concentration of 0.0005 mol / L, soak the pretreated carbon cloth in the solution for 2 hours, and then conduct a hydrothermal reaction at 85°C for 2.5 hours, then wash with deionized water, and dry at 60°C in the air for 8 hours to obtain a carbon cloth Loaded manganese-containing precursor; the KMnO 4 and IrCl 3 Dissolve in deionized water and stir evenly to obtain K + and Ir 3+ The concentrations were 0.001 mol / L and 0.00025 mol / L mixed solution respectively, the above-mentioned dried carbon cloth loaded with manganese precursor was immersed in the mixed solution for 2 h, then hydrothermally reacted at...
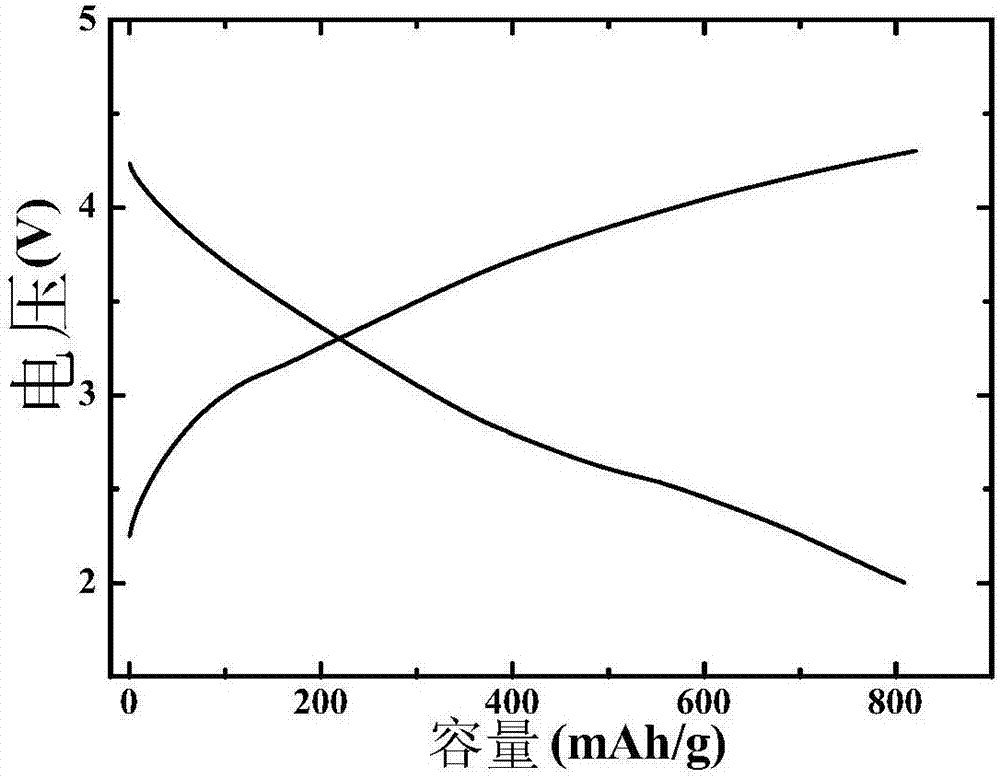
Embodiment 2
[0076] After the commercial carbon cloth was hydrothermally reacted in 69wt% concentrated nitric acid at 90°C for 2 hours, it was first washed with deionized water, then ultrasonically cleaned with acetone, alcohol, and deionized water for 15 minutes, and then heated in air at 60°C. Dry for 8h; KMnO 4 Dissolve in deionized water and stir well to obtain K + A solution with a concentration of 0.0003mol / L, the above pretreated carbon cloth was immersed in the solution for 2 hours, and then hydrothermally reacted at 70°C for 1 hour, then washed with deionized water, and dried at 60°C in the air for 8 hours to obtain a carbon cloth load. The manganese-containing precursor; the KMnO 4 and IrCl 3 Dissolve in deionized water and stir evenly to obtain K + and Ir 3+ The concentrations were 0.0008mol / L and 0.0003mol / L mixed solution respectively, the above dried carbon cloth loaded with manganese precursor was immersed in the mixed solution for 2h, then hydrothermally reacted at 70℃ ...
Embodiment 3
[0080] After the commercial carbon cloth was hydrothermally reacted in 69wt% concentrated nitric acid at 90°C for 2 hours, it was first washed with deionized water, then ultrasonically cleaned with acetone, alcohol, and deionized water for 15 minutes, and then heated in air at 60°C. Dry for 8h; KMnO 4 Dissolve in deionized water and stir well to obtain K + A solution with a concentration of 0.001mol / L, soak the pretreated carbon cloth in the solution for 2 hours, and then react it with water at 90°C for 3 hours, then wash it with deionized water, and dry it in the air at 60°C for 8 hours to obtain a carbon cloth load. The manganese-containing precursor; the KMnO 4 and IrCl 3 Dissolve in deionized water and stir evenly to obtain K + and Ir 3+ The concentrations were 0.0015 mol / L and 0.0002 mol / L mixed solutions respectively. The above-mentioned dried carbon cloth loaded with manganese precursor was immersed in the mixed solution for 2 h, then hydrothermally reacted at 90 ° ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com