Clevidipine butyrate related substance detection method
A clevidipine butyrate and detection method technology, which is applied in the field of drug analysis, can solve problems such as poor separation effect, and achieve the effects of simple method, high sensitivity and specificity, and strong operability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Dissolving sample solvent and concentration selection
[0032] For the choice of solvent for dissolving samples, acetonitrile, methanol, and mobile phase are used to dissolve samples, and they can all be dissolved. To reduce the appearance of solvent peaks, dissolve the sample with the mobile phase.
[0033] When the mobile phase is used as the solvent of the sample, the mobile phase is formulated into three concentrations of 0.2mg / ml, 0.5mg / ml and 1.0mg / ml respectively. Although these three concentrations can achieve the separation of impurities, the detection response value is too small when 0.2 mg / ml is used, and when 1.0 mg / ml is used, the concentration of the mobile phase is too large to be conducive to the separation of the sample, so the final choice is 0.5 mg / ml as the concentration of the test solution.
Embodiment 2
[0035] Adopt American Agilent 1260 high performance liquid chromatograph, autosampler; Welchrom C18 column; Mobile phase volume ratio is acetonitrile: ethanol: water=16:40:44; Injection volume is 20 μ l; Detection wavelength is 240nm; Flow rate is 1.0 ml / min; column temperature is 35°C.
[0036] Experimental steps:
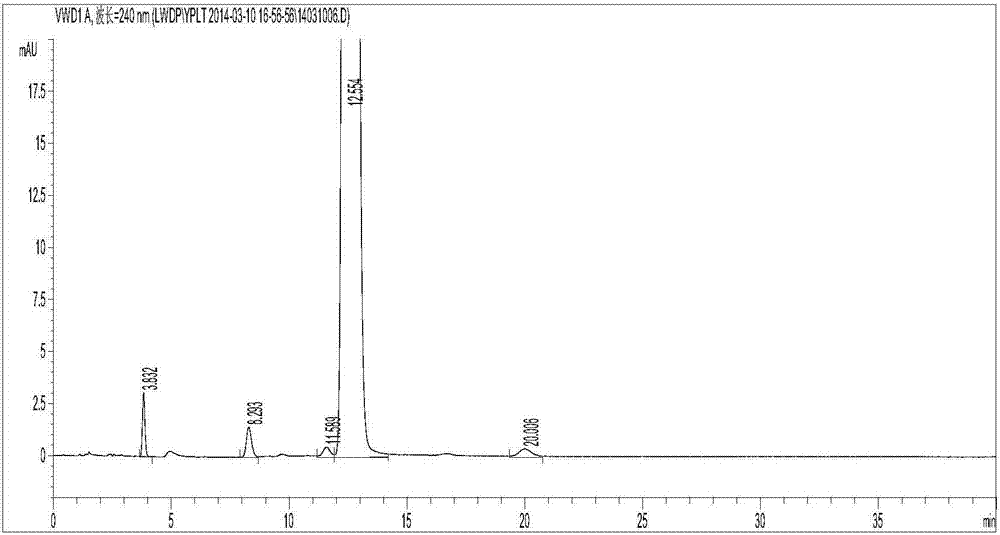
[0037] Get clevidipine butyrate and its impurity Ⅰ, Ⅲ, Ⅳ, Ⅴ amount, dissolve and dilute with mobile phase to make every 1ml contain clevidipine butyrate 0.50mg, impurity 0.5μg solution, shake well, as the test sample solution. Inject and measure according to the above conditions, record the chromatogram, the results are shown in figure 1 . figure 1 It shows that the separation between clevidipine butyrate and its impurities and between impurities and impurities meets the requirements.
Embodiment 3
[0039] Adopt American Agilent 1260 high performance liquid chromatograph, autosampler; WelchromC18 column (4.6 * 250mm, 5 μ m); Mobile phase is methanol: ethanol: water = 15: 45: 40; Injection volume is 20 μ l; Detection wavelength is 240nm ; The flow rate is 1.0ml / min; The column temperature is 40°C.
[0040] Experimental steps:
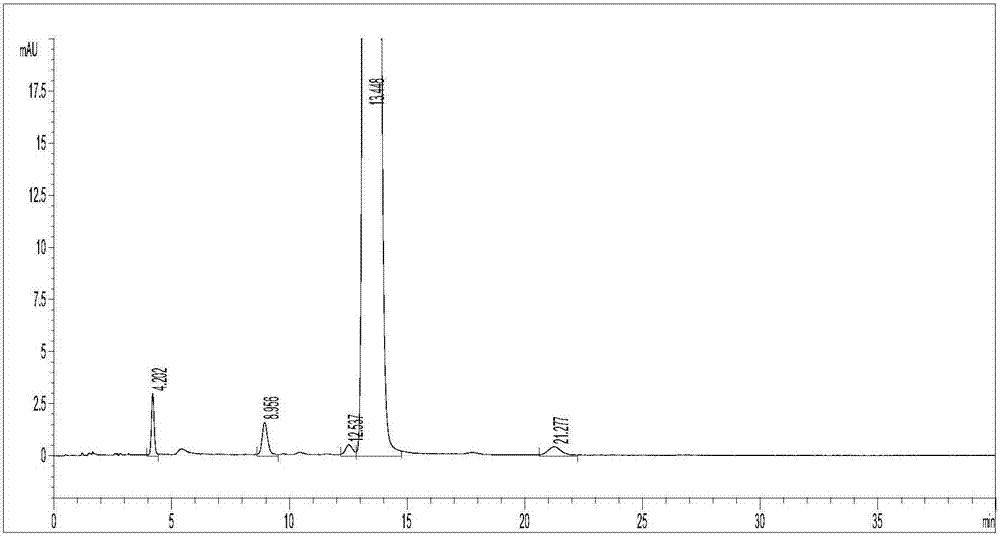
[0041] Get clevidipine butyrate and its impurity Ⅰ, Ⅲ, Ⅳ, Ⅴ amount, dissolve and dilute with mobile phase to make every 1ml contain clevidipine butyrate 0.50mg, impurity 0.5μg solution, shake well, as the test sample solution. Inject and measure according to the above conditions, record the chromatogram, the results are shown in figure 2 . figure 2 It shows that the separation between clevidipine butyrate and its impurities and between impurities and impurities meets the requirements
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com