Application of kavalactone compound in preparing medicine for inhibiting infection of enterovirus 71
A technology of kavasourin and enterovirus, which is applied in antiviral agents, pharmaceutical formulas, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1: Screening of small molecule compounds for high-throughput inhibition of EV-A71 virus-infected cells
[0021] 1. Cells, viruses and compounds
[0022] RD (human rhabdomyomas) cells, Vero (African green monkey kidney) cells and BHK-21 (baby hamster kidney) cells in DMEM medium containing 1% penicillin / streptomycin and 10% fetal bovine serum at 37℃ , Cultivate in a 5% carbon dioxide incubator. The EV-A71 FY573 strain (GenBank accession number HM064456) is used for high-throughput drug screening, and the EV-A71 G082 strain is used for virus plaque reduction experiments and dosing experiments at different time points. EV-A71 virus strain SH12-276 (GenBank accession number KC570453), EV-A71 virus strain SH12-036 (GenBank accession number KC570452), CVA16 SHZH05-1 strain (GenBank accession number EU262658), Coxsackie virus B3 (CVB3, Nancy strain, ATCC VR-30) and type I poliovirus (PV1, Sabin strain) were used in the study of the antiviral spectrum of the compound. The...
Embodiment 2
[0025] Example 2: Determination of the inhibitory effect and cytotoxicity of kavapirin compounds on viruses
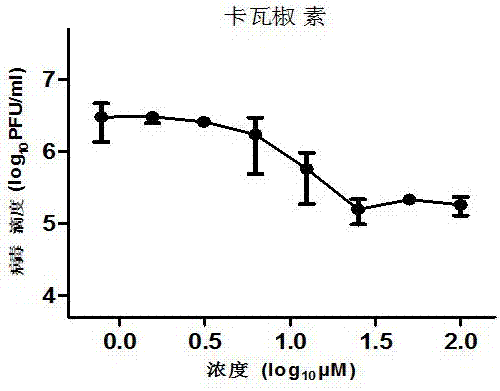
[0026] 1. Perform a virus infection inhibition test on different concentrations of kavakyorin
[0027] RD cells in the logarithmic growth phase are divided by 10 4 Cells / well were added to a 96-well cell culture plate and cultured for 24 hours. RD cells were infected with EV-A71 with a multiplicity of infection of 0.1, and a 3-fold dilution of kavakyorin was added. After 42 hours of culture, the supernatant was collected , Use plaque formation test to determine virus titer.
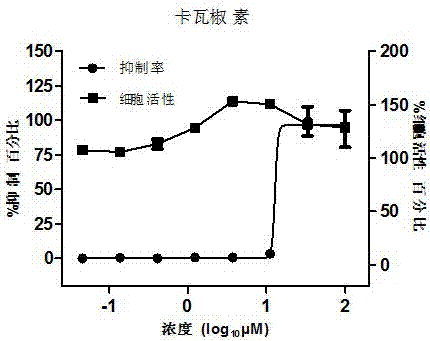
[0028] 2. Perform cytotoxicity experiments on different concentrations of kavakyorin
[0029] The experimental method is the same as the dose-dependent experiment, but no virus liquid is added. After culturing for 42 hours, 50 μl CellTiter-Glo reagent (Promega) was added, and the cell viability was judged by detecting the chemiluminescence intensity.
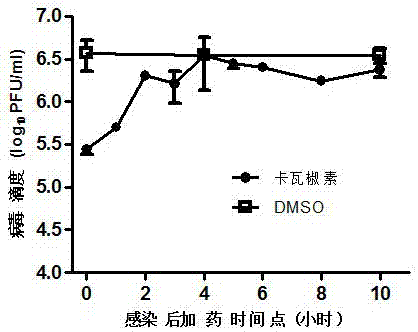
[0030] The results showed that kavatamycin can effect...
Embodiment 3
[0036] Example 3: Determination of virus titer
[0037] To measure the titer of EV-A71, add 1 ml of DMEM containing 3×105 RD cells to each well of a 12-well plate and culture for 24 hours. The virus was diluted by a factor of 10, that is, 25 μl of virus solution was mixed with 225 μl of DMEM containing 2% FBS and 1% penicillin / streptomycin. Aspirate the medium in the 12-well plate and add 200 μl of virus to each well. Place the infection in a 37°C, 5% carbon dioxide incubator for 1 h, and gently shake it every 15 minutes. Then aspirate the virus solution, add 1 ml of DMEM containing 0.8% methylcellulose and 2% fetal bovine serum, incubate for 6 days in a 37°C, 5% carbon dioxide incubator, and place it in 3.7% formalin for 1 h After that, stain with 1% crystal violet. For other viruses, CV-B3 was cultured for 1 day, PV1 was cultured for 2 days, and CV-A16 was cultured for 3 days before staining. The titer of EV-D68 is determined by half the tissue culture infectious dose (TCID...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com