Collagen-based bilayer membrane material for directional release of loaded growth factors and manufacturing method of collagen-based bilayer membrane material
A growth factor and double-layer film technology, which is applied in the fields of pharmaceutical formulation, medical science, prosthesis, etc., can solve the problems of disorganized bioactive delivery or poor controllability of cell targeting and release behavior, and achieve good plasticity and raw material Abundant and easy to obtain, good biocompatibility effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
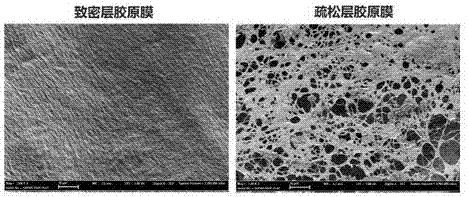
[0032] Embodiment 1: the preparation of dense layer and loose layer collagen film
[0033] 1. Preparation of Bovine Tendon Type Ⅰ Collagen Swelling Solution
[0034] Fully wash the fresh bovine tendon purchased in the market, remove the connective tissue such as the tissue around the tendon, tendon sheath and fascia, and freeze it in a freezer after washing. Take out the frozen bovine tendon and cut it into slices about 1 mm thick, add ficin solution (concentration 0.05-0.25%) to treat it, stir it and place it in an incubator for 24 hours for enzymatic hydrolysis, then add excess H 2 o 2 Terminate the reaction, wash with distilled water 3-4 times, add 0.3% malonic acid solution to swell for 24 h after decanting. The swollen tendons were fully stirred for 6 hours, and the viscosity was adjusted with 0.3% malonic acid solution, filtered with 80-120 mesh stainless steel mesh under positive pressure to remove impurities and unswollen substances, and packaged.
[0035] 2. Prepar...
Embodiment 2
[0047] Example 2: Preparation of dense-loose collagen bilayer membrane
[0048] Add 80 mg / mL fibrinogen solution dropwise to one side of the cross-linked dense layer collagen membrane in the above example, and cover the entire surface; then drip 400 IU / mL thrombin solution to one side of the cross-linked loose layer collagen membrane, Also cover the entire surface; then overlap the two collagen films and glue them together, use the chemical reaction of thrombin and fibrinogen to quickly react to form fibrin glue to bond the collagen double-layer film, and air dry; then use the pressure film Machine flattening, that is, a dense-loose collagen double-layer membrane material. The average thickness of the bilayer film is 1 mm.
Embodiment 3
[0049] Example 3: Cy5.5-NHS ester labeled FGF2
[0050] 1. Weigh 0.46mg of Cy5.5-NHS ester and dissolve it in 100uL dimethyl sulfoxide solution.
[0051] 2. Prepare FGF2 into a 100ug / mL solution with a pH value above 8.0.
[0052] 3. Take 20uL FGF2 solution, add 1uL Cy-5.5-NHS ester solution, mix well, avoid light, and react at room temperature for at least 4 hours.
[0053] 4. Ultrafilter the reaction solution of FGF2 and Cy5.5-NHS ester in an ultrafiltration tube, 10000 rpm, 20 minutes, ultrafiltration 8 times, the reaction solution in the ultrafiltration tube turns from blue to colorless, collect the ultrafiltration tube medium reaction solution.
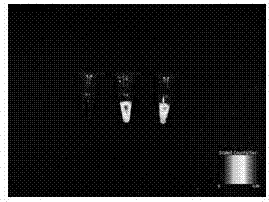
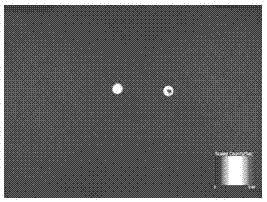
[0054] 5. For the detection of Cy-5.5-labeled FGF2 in the in vivo imager, see figure 2 .
[0055] 6. The FGF2 solution labeled Cy5.5 was added dropwise to the side of the loose layer in the dense-loose collagen bilayer membrane of Example 2, and it was observed in the live imager that the FGF2 solution would not penetr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Outer wall diameter | aaaaa | aaaaa |
| Inner diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com