A kind of phloroglucinol derivative and its application in the preparation of antibacterial drugs
A technology of phloroglucinol and antibacterial drugs, applied to phloroglucinol derivatives and the application field in the preparation of antibacterial drugs, can solve problems such as lack of in-depth research, and achieve low eukaryotic cell toxicity and significant antibacterial activity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
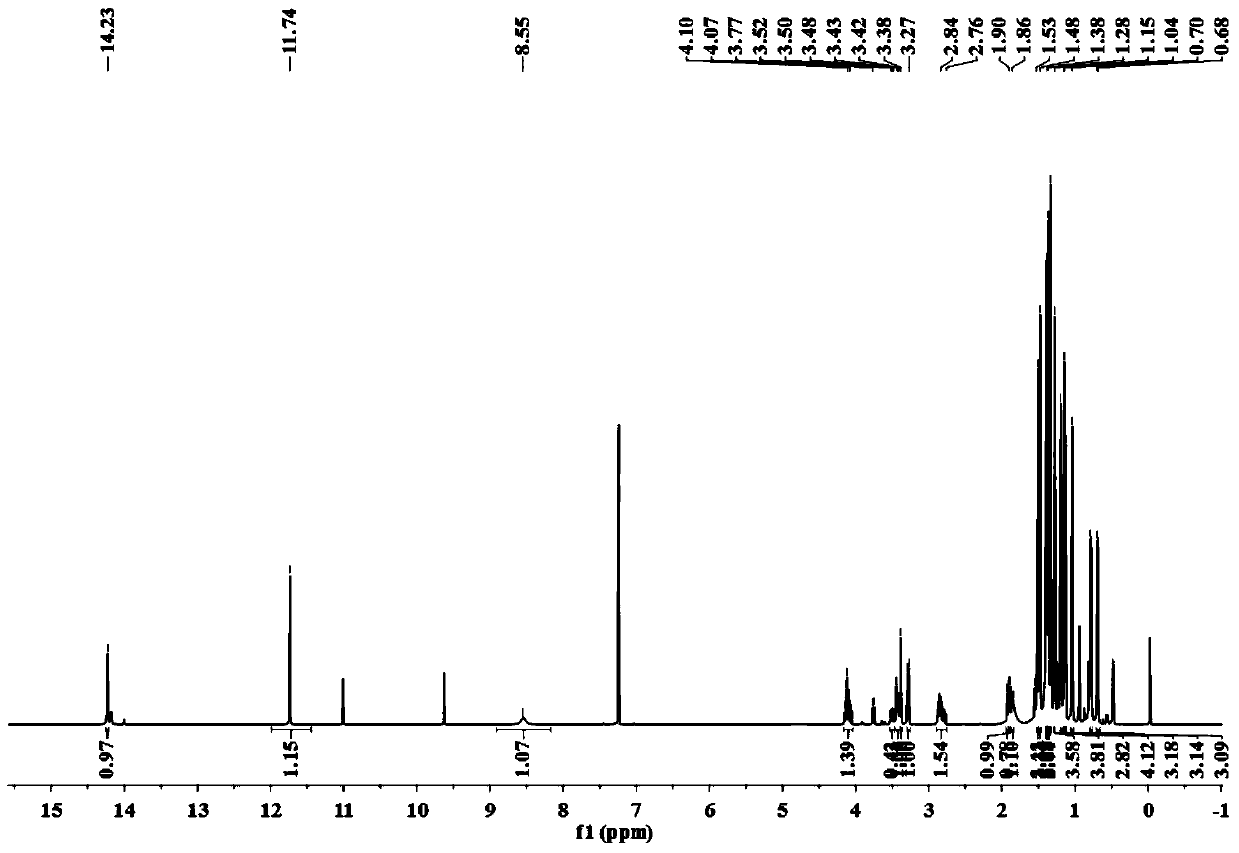
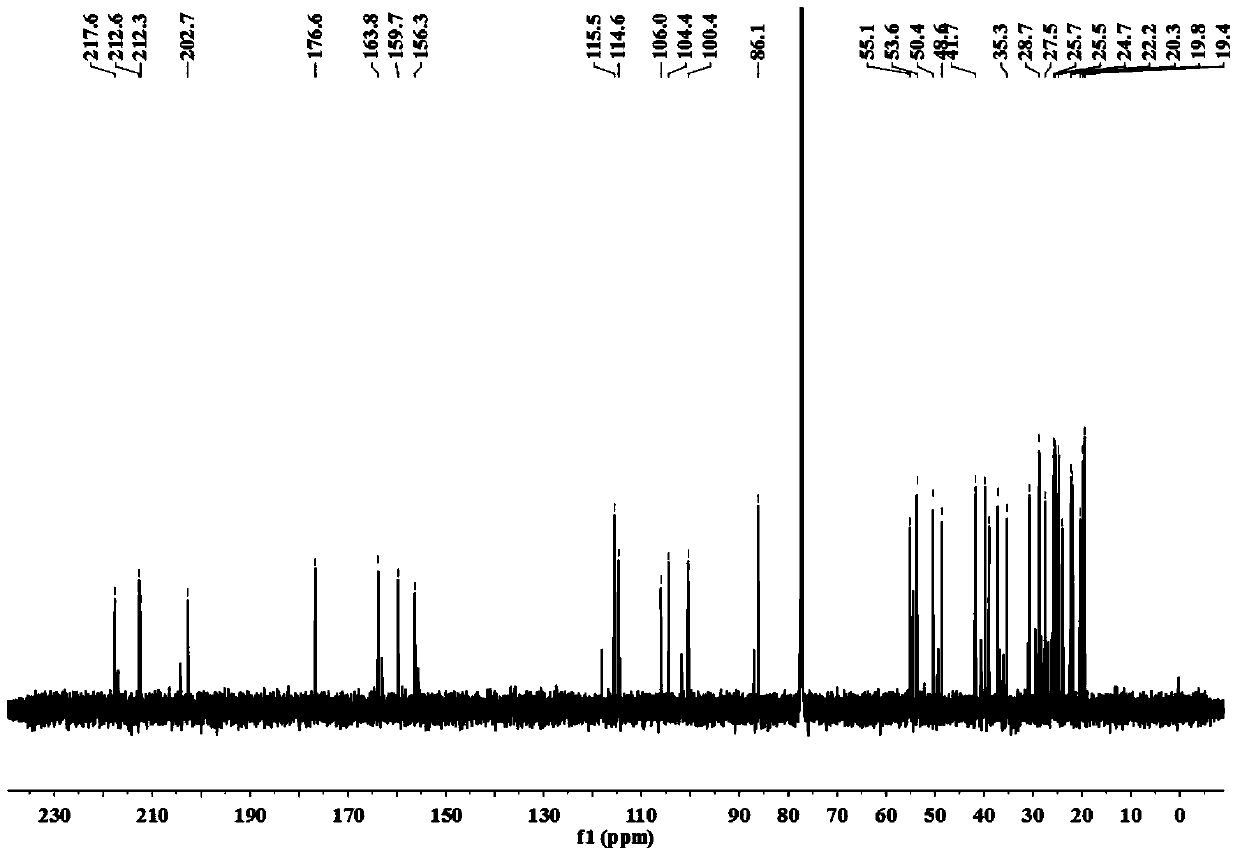
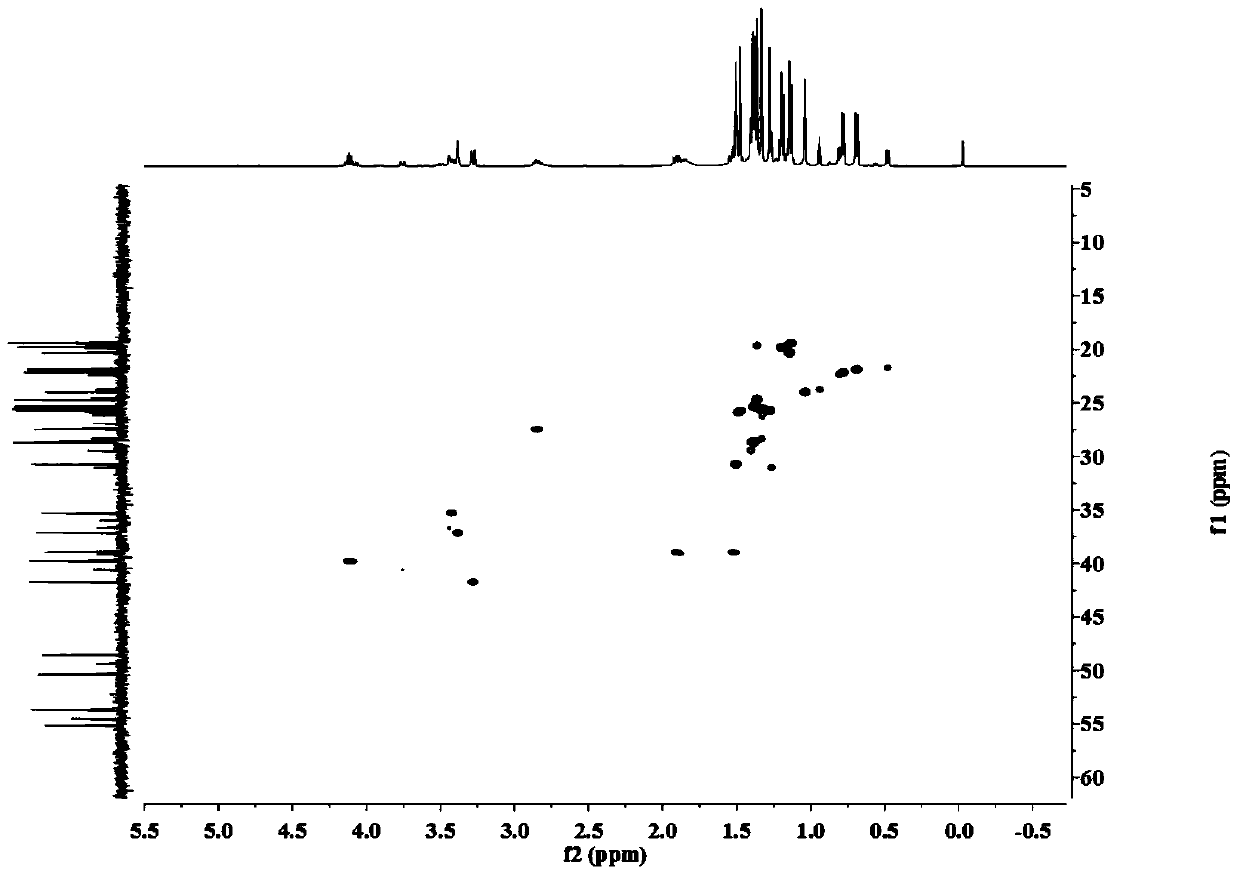
[0029] The separation, extraction and structural identification of phloroglucinol derivatives comprises the following steps:
[0030] (1) Weigh 8 kg of dried myrtle (Myrtus communis Linn.) branches and leaves, pulverize into a coarse powder with a pulverizer, extract 4 times with 95% (w / w) ethanol percolation, 25 L each time, combine the percolation liquid and decompress Concentrated to no alcohol smell, the total extract obtained is about 1.2kg. The extract was suspended with water and then extracted with petroleum ether to obtain 389 g of petroleum ether extraction fractions.
[0031] (2) Carry out silica gel column chromatography on the petroleum ether extraction part, using petroleum ether-ethyl acetate as the eluent, according to the volume ratio of petroleum ether and ethyl acetate: 100:0, 100:1, 100:3, 100:5 , 100:7, 100:10, 100:30, 100:50, 100:100 and 0:100 elution gradients were eluted, analyzed by thin layer chromatography (TLC) and combined similar fractions to obt...
Embodiment 2
[0048] Inhibitory effects of (±) Myrtucyclitone C and (±) Myrtucyclitone D on various bacteria
[0049] Staphylococcus aureus S.aureus ATCC29213, methicillin-resistant Staphylococcus aureus S.aureusATCC33591 (MRSA), vancomycin-intermediate resistant Staphylococcus aureus S.aureus Mu50 (VISA), Staphylococcus epidermidis S.epidermidis ATCC12228, E.faecalis ATCC29212, E.faecium 13-01, Escherichia coli ATCC25922, Ps.Aeruginosa Ps.Aeruginosa, the above strains were purchased from the Institute of Pharmaceutical Biotechnology, Chinese Academy of Medical Sciences.
[0050] The minimum inhibitory concentration (MIC) of the compound's antibacterial effect in vitro was determined by the broth micro-doubling dilution method, and the specific operation method was as follows:
[0051] (1) Bacterial culture: Cultivate the experimental bacteria with Mueller-Hinton (MH) broth medium, when it grows for 8-12h to about 0.5 Mcfarland concentration (1 × 10 8 CFU) for backup.
[0052] (2) Dissolv...
Embodiment 3
[0061] Effects of (±) Myrtucyclitone C and (±) Myrtucyclitone D on normal cells
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com