(2-(substituted phenyl)indenyl)-bis(3,5-bis(trifluoromethyl))phenyl phosphine ligand and preparation method thereof
A technology of trifluoromethyl and phenylphosphine, which is applied in the field of organochemical metal-catalyzed ligand synthesis, can solve the problems of limited application, unfavorable reduction and elimination of electron-rich ligands, etc., and achieves simple operation, rich synthesizable structures, and easy raw materials The effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
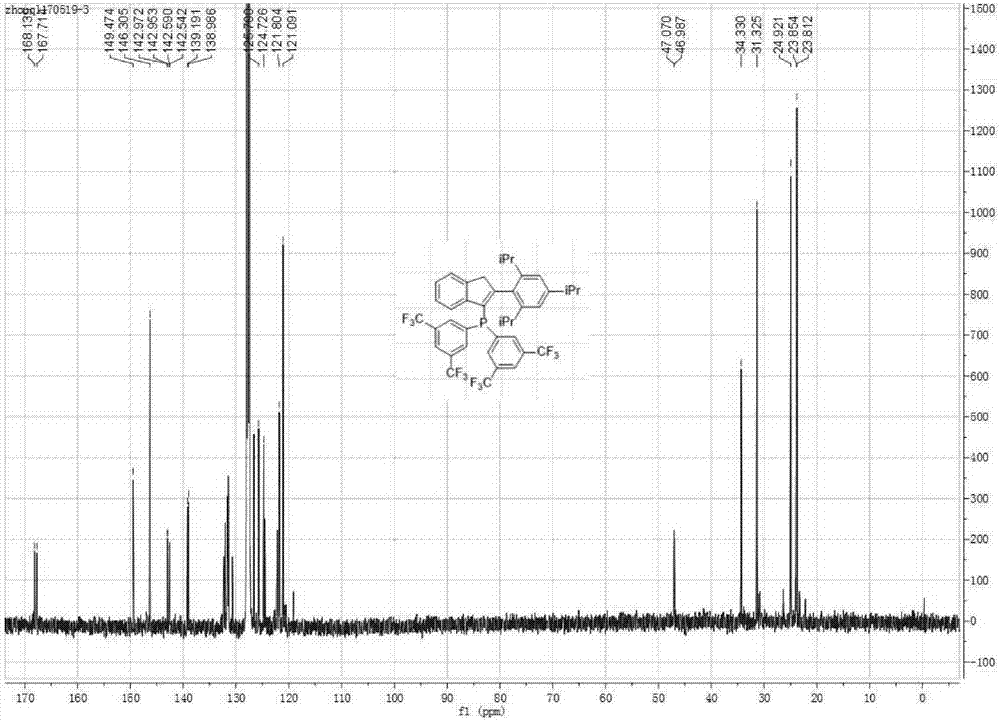
[0082] Preparation of (2-(2,4,6-triisopropylphenyl)indenyl)-bis(3,5-bis(trifluoromethyl))phenylphosphine
[0083] a) Preparation of 2-(2,4,6-triisopropylphenyl)indene: 3 mmol of 2-bromoindene (Shanghai Beide Pharmaceutical Technology Co., Ltd.) , 1.5mmol of 2,4,6-triisopropylphenylboronic acid (Shanghai Beide Pharmaceutical Technology Co., Ltd.), 3mmol% of palladium acetate, 6mmol% of S-Phos and 3mmol of potassium phosphate, add 20mL of tetrahydrofuran and 10mL of water The mixture to dissolve the reactants was reacted at 100°C for 24 hours. After cooling, the reaction solution was extracted with ethyl acetate, the organic phase was dried with anhydrous sodium sulfate, the organic phase solvent was removed, and column chromatography was carried out with petroleum ether as a developing solvent (Rf=0.8) to obtain 2-(2,4,6- Triisopropylphenyl) indene, the yield is 85%.
[0084] b) Preparation of (2-(2,4,6-triisopropylphenyl)indenyl)-bis(3,5-bis(trifluoromethyl))phenylphosphine:...
Embodiment 2
[0086] Preparation of (2-(2,4,6-trimethylphenyl)indenyl)-bis(3,5-bis(trifluoromethyl))phenylphosphine
[0087] a) Preparation of 2-(2,4,6-trimethylphenyl)indene: under an argon atmosphere, in a Schlenk bottle, 3 mmol of 2-bromoindene (Shanghai Biide Pharmaceutical Technology Co., Ltd.), 1.5mmol of 2,4,6-trimethylphenylboronic acid (Shanghai Beide Pharmaceutical Technology Co., Ltd.), 3mmol% of tetrakistriphenylphosphine palladium, 6mmol% of S-Phos and 3mmol of potassium phosphate, add 20mL of toluene and 10 mL of water to dissolve the reactants. React at 100°C for 24 hours, extract the reaction solution with ethyl acetate after cooling, dry the organic phase with anhydrous sodium sulfate, remove the organic phase solvent, and perform column chromatography with petroleum ether as a developing solvent (Rf=0.8) to obtain 2 -(2,4,6-trimethylphenyl)indene, the yield is 90%.
[0088]b) Preparation of (2-(2,4,6-trimethylphenyl)indenyl)-bis(3,5-bis(trifluoromethyl))phenylphosphine: ...
Embodiment 3
[0090] Preparation of (2-(2,6-dimethoxyphenyl)indenyl)-bis(3,5-bis(trifluoromethyl))phenylphosphine
[0091] a) Preparation of 2-(2,6-dimethoxyphenyl)indene: under an argon atmosphere, in a Schlenk bottle, 3 mmol of 2-bromoindene (Shanghai Beide Pharmaceutical Technology Co., Ltd.), 1.5 Mmol of 2,6-dimethoxyphenylboronic acid (Shanghai Beide Pharmaceutical Technology Co., Ltd.), 3mmol% of palladium acetate, 6mmol% of S-Phos and 3mmol of potassium phosphate were added to a mixture of 20mL THF and 10mL water to dissolve Reactant. React at 100°C for 24 hours, extract the reaction solution with ethyl acetate after cooling, dry the organic phase with anhydrous sodium sulfate, remove the organic phase solvent to dryness, and perform column chromatography with petroleum ether as a developing solvent (Rf=0.8) to obtain 2 -(2,6-dimethoxyphenyl)indene, the yield is 79%.
[0092] b) Preparation of (2-(2,6-dimethoxyphenyl)indenyl)-bis(3,5-bis(trifluoromethyl))phenylphosphine: under argo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com