Allisartan isoproxil pharmaceutical composition, preparation containing pharmaceutical composition and preparation method of pharmaceutical composition
A technology of alisartan medoxomil and its composition, which is applied in the field of pharmaceutical preparations, can solve the problems of high tablet weight, low load of alisartan medoxomil preparations, and insufficient support for industrial production, and achieve low production costs and strong process controllability , the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
0.05
[0068] (1) Dissolve the prescription amount HPMC E5 in water to prepare a 3mg / mL aqueous solution;
[0069] (2) add recipe quantity alisartan medoxomil to step (1) gained aqueous solution, airtight container, reduce container internal pressure to 20Kpa, stir and make it be suspended in aqueous phase and return to normal pressure;
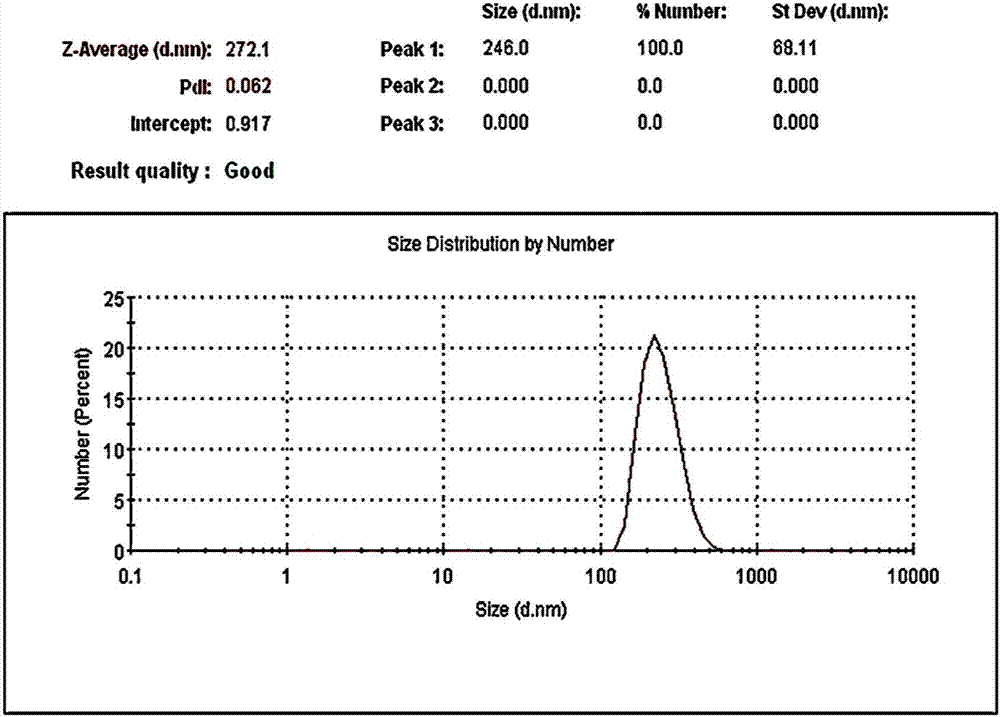
[0070] (3) Add 0.1mm ball milling beads to the suspension obtained in step (2), and grind alisartan medoxomil to an average particle size value of 272.1nm (PDI=0.062), and the micrograph of the resulting nanosuspension is as follows figure 1 As shown, the particle size distribution diagram is shown in figure 2 shown;
[0071] (4) Add half of the volume of the suspension to the suspension obtained in step (3) and the protective agent of the prescribed amount to obtain the alisartan medoxomil pharmaceutical composition (suspension);
[0072] (5) Spray drying the alisartan medoxomil pharmaceutical composition (suspension) obtained in st...
Embodiment 2
0.02
[0076] (1) Dissolve the prescription amount HPMC E50 in water to prepare an aqueous solution of 1.5 mg / mL;
[0077] (2) Add prescription amount of alisartan medoxomil to step (1) gained aqueous solution, airtight container, reduce container internal pressure to 20Kpa, stir and recover normal pressure after making it be suspended in aqueous phase;
[0078] (3) Add 1.5 mm ball milling beads to the suspension obtained in step (2), and replace it with 1.0 mm ball milling beads after grinding for 45 minutes, and grind alisartan medoxomil to an average particle size of 515.1 nm (PDI=0.237);
[0079] (4) Add half of the volume of the suspension to the suspension obtained in step (3) and the protective agent of the prescribed amount to obtain the alisartan medoxomil pharmaceutical composition (suspension);
[0080] (5) Spray drying the alisartan medoxomil pharmaceutical composition (suspension) obtained in step (4) to obtain the alisartan medoxomil pharmaceutical composit...
Embodiment 3
0.05
[0084] (1) Dissolve the prescription amount of PVP K30 in water to prepare a 0.8 mg / mL aqueous solution;
[0085] (2) Add prescription amount of alisartan medoxomil to step (1) gained aqueous solution, airtight container, reduce container internal pressure to 20Kpa, stir and recover normal pressure after making it be suspended in aqueous phase;
[0086] (3) Add 1.0 mm ball milling beads to the suspension obtained in step (2), and replace it with 0.2 mm ball milling beads after grinding for 45 minutes, and grind alisartan medoxomil to an average particle size of 365.4 nm (PDI=0.222);
[0087] (4) Add half of the volume of the suspension to the suspension obtained in step (3) and the protective agent of the prescribed amount to obtain the alisartan medoxomil pharmaceutical composition (suspension);
[0088] (5) Spray drying the alisartan medoxomil pharmaceutical composition (suspension) obtained in step (4) to obtain the alisartan medoxomil pharmaceutical compositio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com