A kind of synthetic method of 2-bromo-1,1,2,2-tetrafluoroethyl substituted aryl building blocks
A tetrafluoroethyl, synthetic method technology, applied in the field of synthesis of aryl building blocks, to achieve the effects of high reaction efficiency, simple and easy access to raw materials and reagents, and a wide range of applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
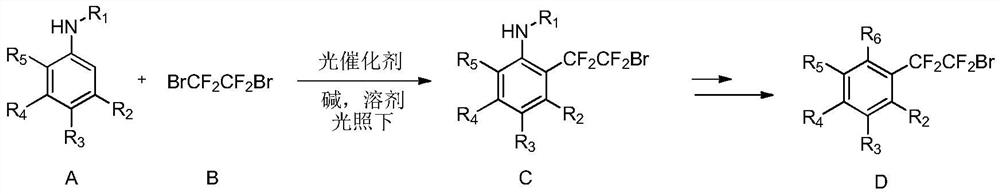
[0033] The invention provides a method for synthesizing dibromotetrafluoroethane-substituted aniline and its derivatives. Preferably, the method includes the step of: in an organic solvent, at a certain temperature (such as 0°C-80°C; preferably 10°C-50°C), under the irradiation of blue light or green light in visible light, to contain iridium , the complex of ruthenium is a photocatalyst, and the compound of formula A (i.e. aniline or its derivatives) is reacted with the compound of formula B) for a period of time (such as 1 to 40 hours) to form the compound of formula C (dibromotetrafluoroethane substituted Aniline and derivatives thereof), and D compounds can be obtained through some classic organic reactions;
[0034]
[0035] In various forms, R 1 , R 2 , R 3 , R 4 , R 5 , R 6 Defined as above.
[0036] More preferably, the compound of formula A is a compound selected from the group consisting of:
[0037]
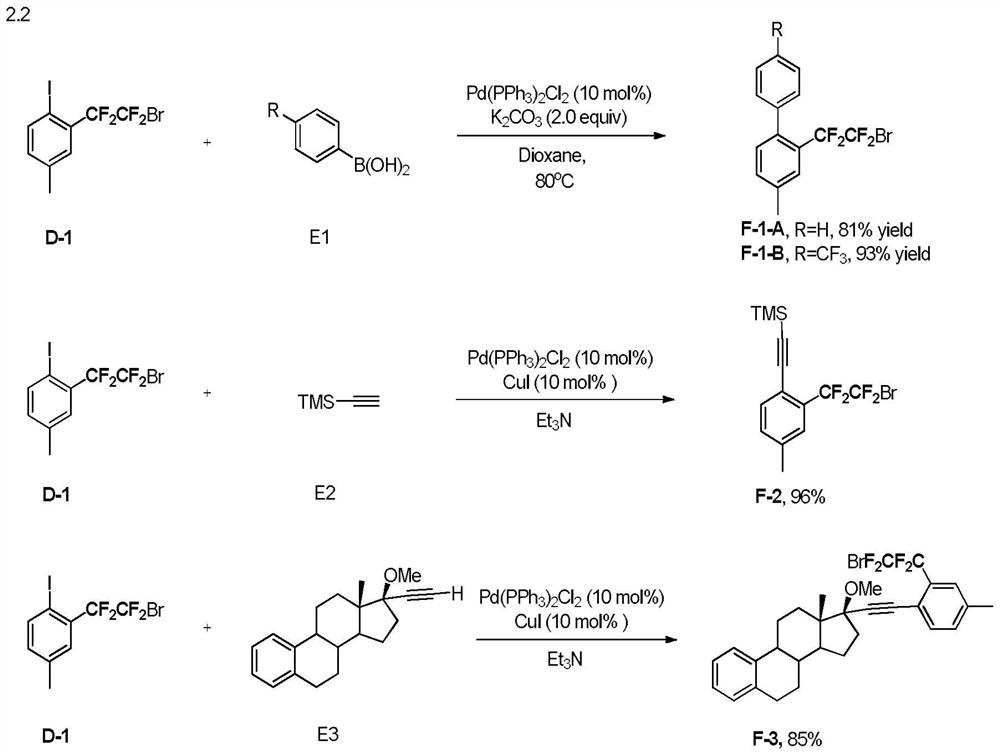
[0038] Compounds of formula A and formula B of the ...
Embodiment 1
[0050]
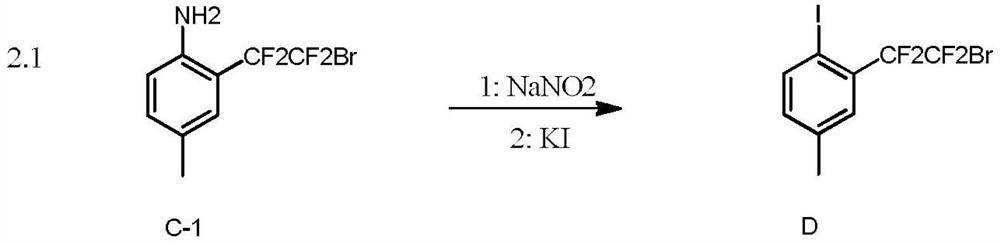
[0051] To a 25mL reaction tube, add 1.3mg (0.5mol%) Ir(PPy), Na 2 CO 3 (0.4mmol), compound A-1 (102mmol, 3 equivalents), after argon replacement three times, add 2mL of acetonitrile (MeCN), inject 50μL (0.40mmol) of compound B-1, and stir for 24 hours under blue light irradiation to obtain compound C-1, 78% yield. 1 HNMR (400MHz, CDCl 3 )δ7.13(1H), 7.10(d, J=8.4Hz, 1H), 6.62(d, J=8.4Hz, 1H), 3.81(br, 2H), 2.26(s, 3H). 19 FNMR (376MHz, CDCl 3 ) δ - 64.5 (t, J = 5.6Hz, 2F), -105.4 (t, J = 5.6Hz, 2F).
Embodiment 2
[0053]
[0054] Into a 25mL reaction tube, add 1.3mg (0.5mol%) Ir(PPy) 3 , K 3 PO 4 (0.4mmol), compound A-1 (1.2mmol, 3 equivalents), nitrogen replacement three times, adding 2mL of acetonitrile (MeCN), injecting 50μL (0.40mmol) of compound B-1, and stirring for 24 hours under blue light irradiation, to obtain compound C-1, 65% yield. 1 HNMR (400MHz, CDCl 3 )δ7.13(1H), 7.10(d, J=8.4Hz, 1H), 6.62(d, J=8.4Hz, 1H), 3.81(br, 2H), 2.26(s, 3H). 19 FNMR (376MHz, CDCl 3 ) δ - 64.5 (t, J = 5.6Hz, 2F), -105.4 (t, J = 5.6Hz, 2F).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com