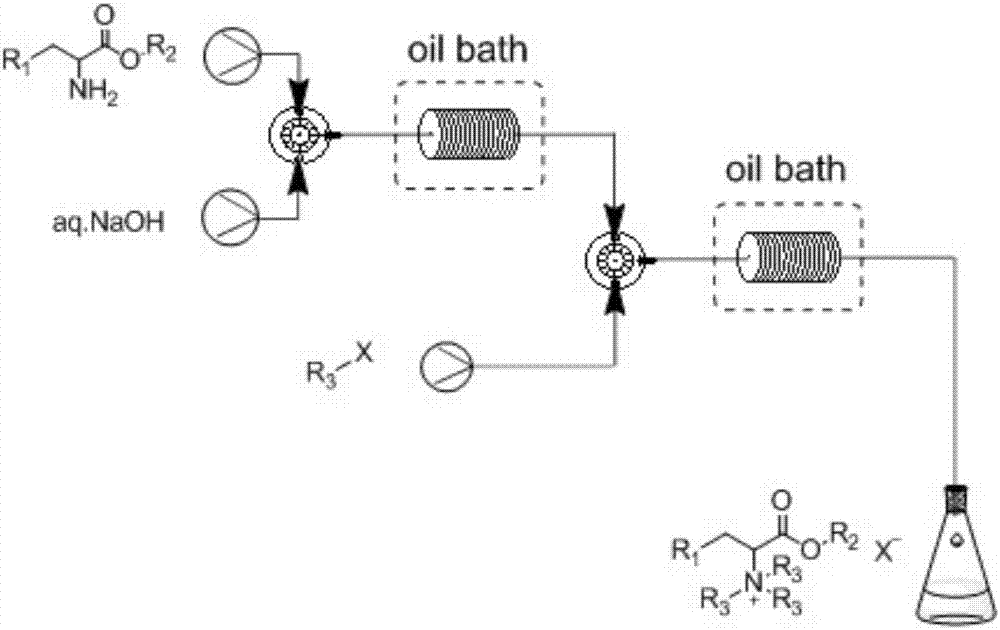
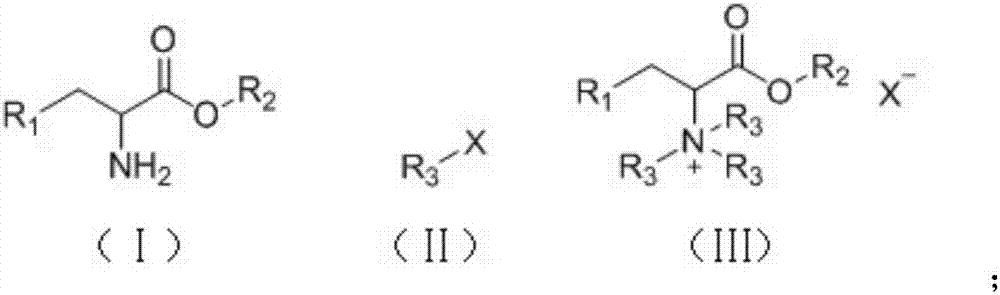
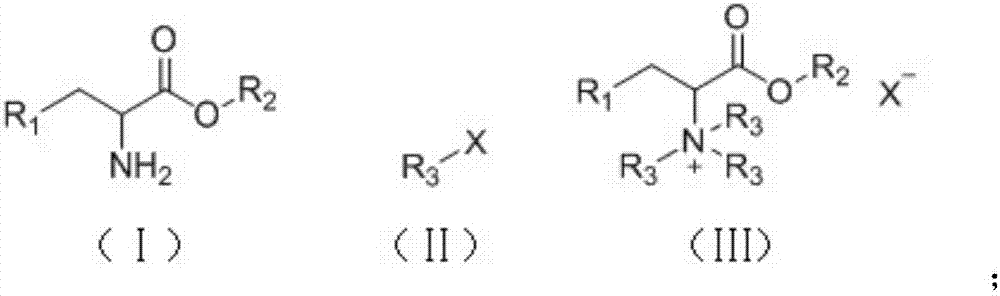
Method of continuously synthesizing quaternary ammonium salt by using microreaction device
A technology of micro-reaction device and reaction device, which is applied in the direction of cyanide reaction preparation, chemical instruments and methods, botany equipment and methods, etc., can solve the problems of unavoidable side reactions, inaccurate control of reaction temperature, cumbersome reaction steps, etc. To shorten the reaction time, simplify the process and improve the reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Take 50g / 100mL of methyl iodide in toluene, 20wt% sodium hydroxide aqueous solution and 40g / 100mL of phenylalanine methyl in toluene, suck them into syringes A, B, and C respectively, and fix them on the syringe pump. Mix the toluene solution of phenylalanine methyl ester and sodium hydroxide aqueous solution through a Y-type mixer with a syringe pump, preheat the resulting mixture to 80°C, and then mix it with the toluene solution of methyl iodide through a bayer mixer , pumped into the microreactor for reaction. The specification of the polytetrafluoroethylene coil as the microreactor is 1mm / 20mL. The molar ratio of phenylalanine methyl ester, sodium hydroxide, and methyl iodide is 1:2:3.3, and the flow rate ratio is 1:0.73:2.1. The specific flow rate is 0.5mL / min for phenylalanine methyl ester, and 0.365 mL / min for sodium hydroxide mL / min, methyl iodide 1.05mL / min. The microreactor was placed in an oil bath to control the temperature, the reaction temperature was k...
Embodiment 2
[0036]Take 50g / 100mL methyl iodide in toluene, 20wt% sodium hydroxide aqueous solution and 40g / 100mL ethyl phenylalanine in toluene, suck them into syringes A, B and C respectively, and fix them on the syringe pump. Mix the toluene solution of ethyl phenylalanine and sodium hydroxide solution through a Y-type mixer with a syringe pump, preheat the resulting mixture to 80°C, and then mix it with the toluene solution of methyl iodide through a bayer mixer , pumped into the microreactor for reaction. The specification of the polytetrafluoroethylene coil as the microreactor is 1mm / 20mL. The molar ratio of ethyl phenylalanine, sodium hydroxide, and methyl iodide is 1:2:3.3, and the flow rate ratio is 1:0.68:1.98. The specific flow rate is ethyl phenylalanine 0.5mL / min, sodium hydroxide 0.34 mL / min, methyl iodide 0.99mL / min. The microreactor was placed in an oil bath to control the temperature, the reaction temperature was kept at 90°C, and the reaction retention time was 11 minut...
Embodiment 3
[0038] Take 50g / 100mL methyl iodide in toluene solution, 20wt% sodium hydroxide aqueous solution and 40g / 100mL phenylalanine propyl ester in toluene solution, draw them into syringes A, B and C respectively, and fix them on the syringe pump. First mix the toluene solution of propyl phenylalanine and sodium hydroxide solution through a Y-type mixer through a syringe pump, and the resulting mixture is preheated to 80°C, and then mixed with the toluene solution of methyl iodide through a bayer mixer , pumped into the microreactor for reaction. The specification of the polytetrafluoroethylene coil as the microreactor is 1mm / 20mL. The molar ratio of propyl phenylalanine, sodium hydroxide, and methyl iodide is 1:2:3.3, and the flow rate ratio is 1:0.63:1.82. The specific flow rate is 0.5 mL / min for propyl phenylalanine, and 0.315 mL / min for sodium hydroxide. mL / min, methyl iodide 0.91mL / min. The microreactor was placed in an oil bath to control the temperature, the reaction temper...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com