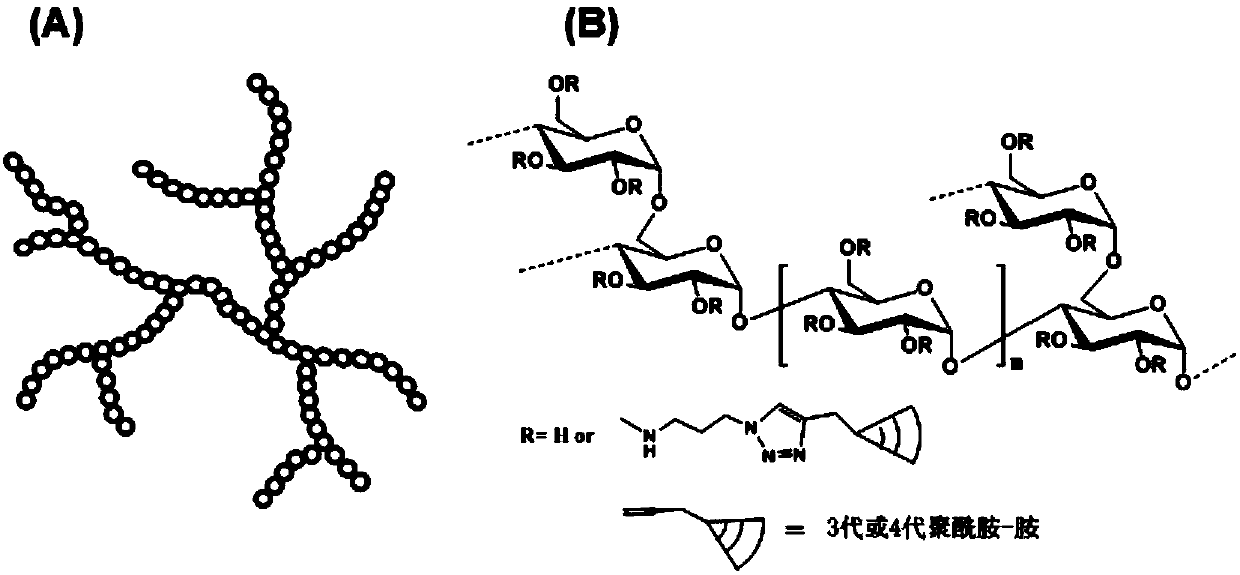
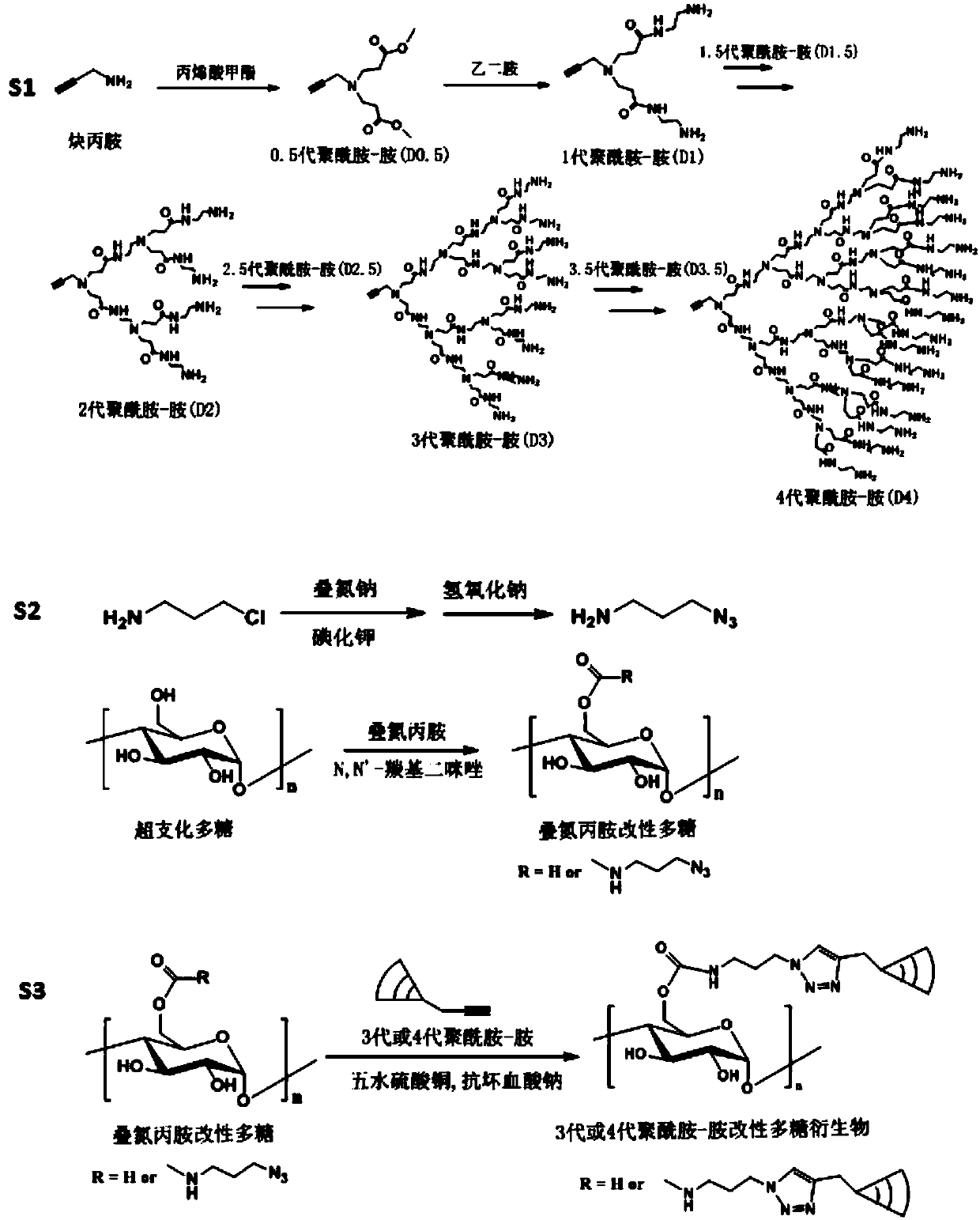
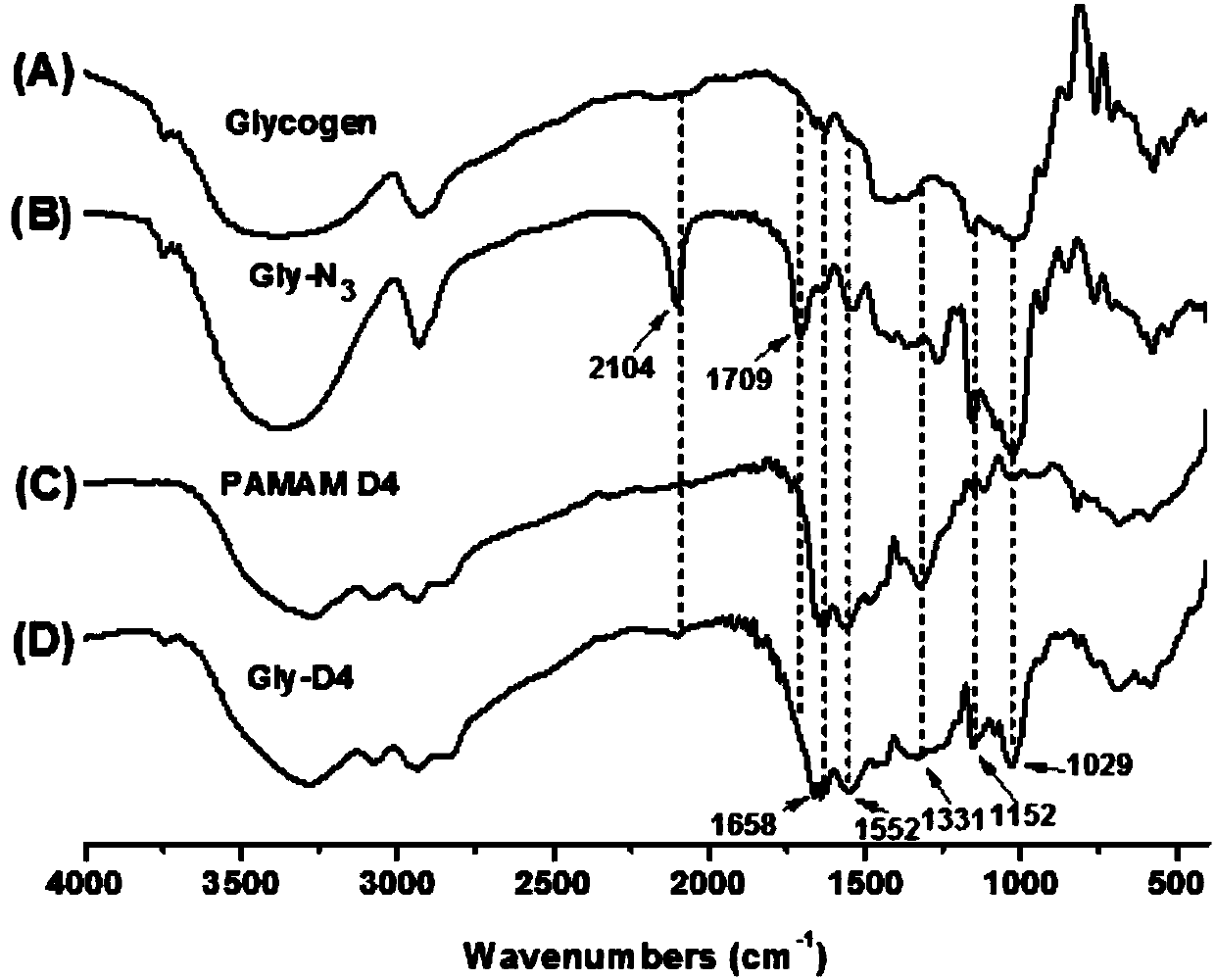
Highly-hyperbranched cationic polysaccharide derivative containing dendritic polyamidoamine group and preparation method of highly-hyperbranched cationic polysaccharide derivative
A technology of cationic polysaccharide and polyamide, which is applied in the field of biomedical engineering and can solve problems such as the difficulty of highly hyperbranched cationic polysaccharide derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] 1. Preparation of highly hyperbranched cationic glycogen derivatives (Gly-D4) modified by 4th generation polyamide-amine
[0070] (1) Nitrogen protection in an ice-water bath, add 10 ml of methanol solution containing 4 g of propargylamine dropwise to 30 ml of methanol solution containing 13.6 g of methyl acrylate, stir for 3 hours, heat up to room temperature and stir for 24 hours, rotate Evaporate and dry in vacuo to obtain 0.5 generation polyamidoamine (D0.5); ice-water bath with nitrogen protection, 100 ml of methanol solution containing 16.2 g of 0.5 generation polyamidoamine was added dropwise to 100 ml of ethylenediamine containing 50 g In ml of methanol solution, stirred for 3 hours, heated to room temperature and stirred for 24 hours, 30°C rotary evaporation, 30°C vacuum-dried to obtain the first-generation polyamide-amine (D1);
[0071] (2) Nitrogen protection in an ice-water bath, 50 ml of methanol solution containing 18 g of 1st generation polyamide-amine wa...
Embodiment 2
[0085] 1. Preparation of highly hyperbranched cationic glycogen derivatives (Gly-D3) modified by 3rd generation polyamide-amine
[0086] (1) Nitrogen protection in an ice-water bath, drop 5 ml of methanol solution containing 0.1 g of propargylamine into 10 ml of methanol solution containing 1 g of methyl acrylate, stir for 2 hours, warm to room temperature and stir for 24 hours, 40 Rotary evaporation at ℃, vacuum drying at 40℃ to obtain 0.5-generation polyamidoamine (D0.5); nitrogen protection in ice-water bath, 15 ml methanol solution containing 1.2 g 0.5-generation polyamido-amine was added dropwise to 6 g of B Diamine in 20 ml of methanol solution, stirred for 2 hours, heated to room temperature, stirred for 24 hours, 40°C rotary evaporation, 40°C vacuum-dried to obtain 1st generation polyamide-amine (D1);
[0087] (2) Nitrogen protection in an ice-water bath, add 20 ml of methanol solution containing 2 grams of 1st generation polyamide-amine dropwise to 25 ml of methanol s...
Embodiment 3
[0093] 1. Preparation of highly hyperbranched cationic glycogen derivatives (Gly-D4) modified by 4th generation polyamide-amine
[0094] (1) Nitrogen protection in an ice-water bath, add 10 ml of methanol solution containing 1.5 g of propargylamine dropwise to 30 ml of methanol solution containing 12 g of methyl acrylate, stir for 1 hour, heat up to room temperature and stir for 24 hours, rotate Evaporate and dry in vacuo to obtain 0.5-generation polyamidoamine (D0.5); under nitrogen protection in an ice-water bath, 30 ml of methanol solution containing 5 g of 0.5-generation polyamido-amine was added dropwise to 50 ml of 15 g of ethylenediamine In methanol solution, stirred and reacted for 1 hour, heated to room temperature and stirred for 24 hours, 60°C rotary evaporation, 60°C vacuum-dried to obtain 1st generation polyamide-amine (D1);
[0095](2) Nitrogen protection in an ice-water bath, dropwise add 30 ml of methanol solution containing 6 g of 1st generation polyamide-amin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com