Application of thyroxine receptor analogues and salts or predrugs thereof to preparation of pulmonary disease treating and/or preventing drugs
A thyroxine and lung disease technology, applied in the field of thyroxine receptor analogs and their pharmaceutically acceptable salts or prodrugs, to achieve the effects of changing the environment of canceration, facilitating removal, and preventing and treating lung diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Preparation of thyroxine receptor analogs:
[0077] Take GC-1, dissolve it in dimethyl sulfoxide, and then dilute it with normal saline to make a liquid for injection and gavage. The working volume is 200 microliters.
[0078] The specific implementation of the present invention will be described below in conjunction with the accompanying drawings.
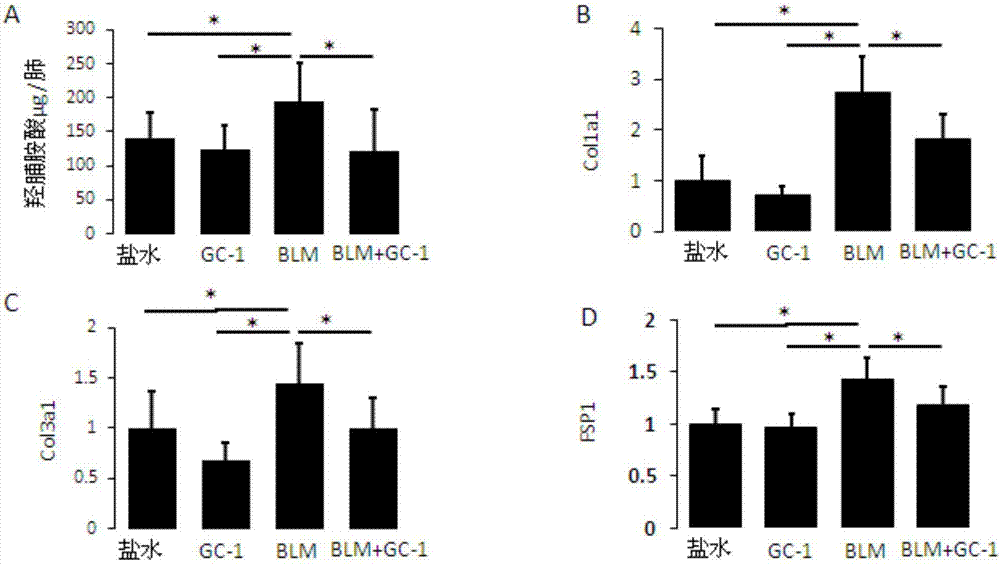
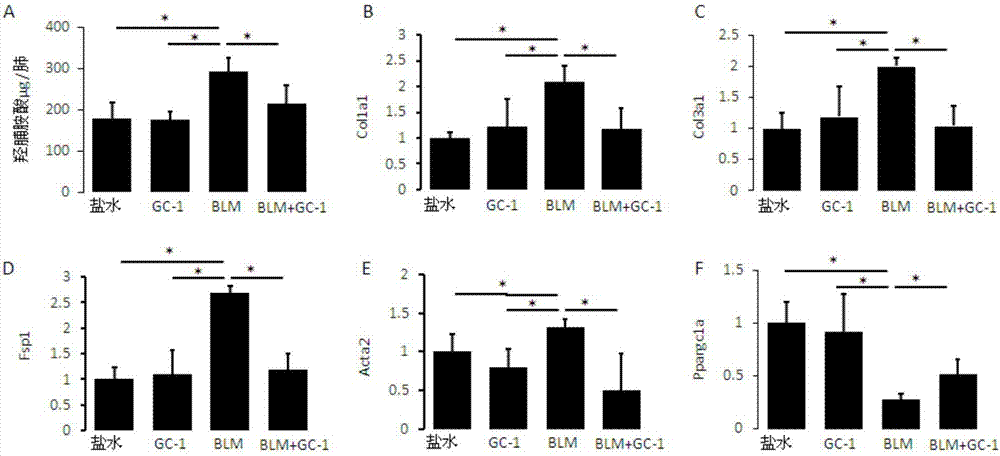
[0079] figure 1 Histogram of hydroxyproline content (A) and gene expression analysis graph (B, C, D) of mice after intraperitoneal injection of GC-1;
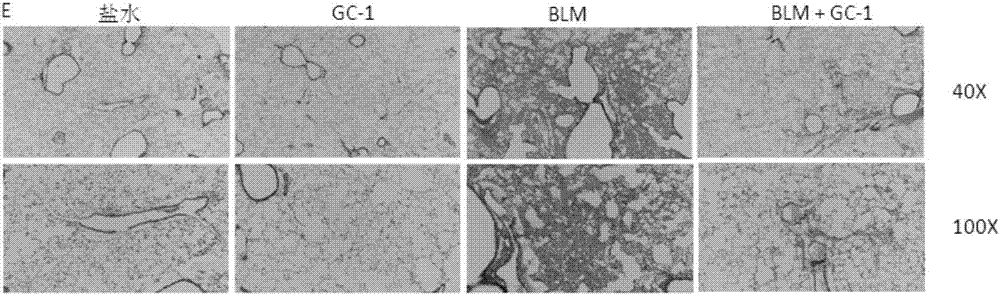
[0080] figure 2 Mason's trichrome staining histochemical analysis diagram of mouse lung tissue after intraperitoneal injection of GC-1;
[0081] figure 1 and figure 2 The therapeutic effect of intraperitoneal injection of GC-1 on pulmonary fibrosis in mice is shown. C57BL / 6 mice were induced by bleomycin (1.5U / kg) for 10 days, and then injected with the thyroxine receptor analogue GC-1 (indicated by BLM+GC-1 in the figure) after the appearance of fibrosis , once a ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com