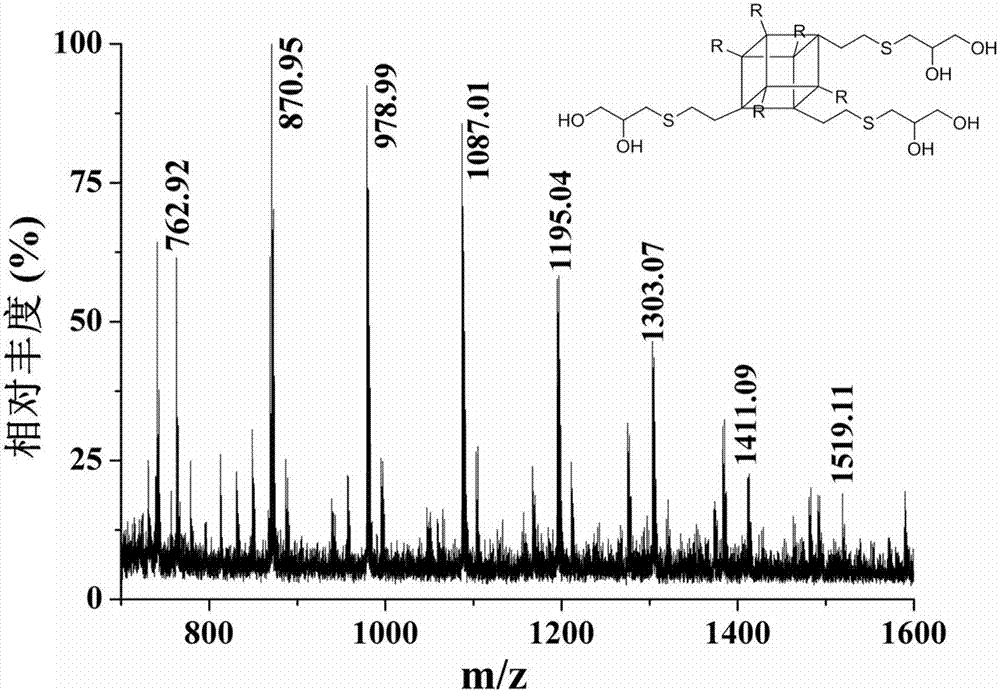
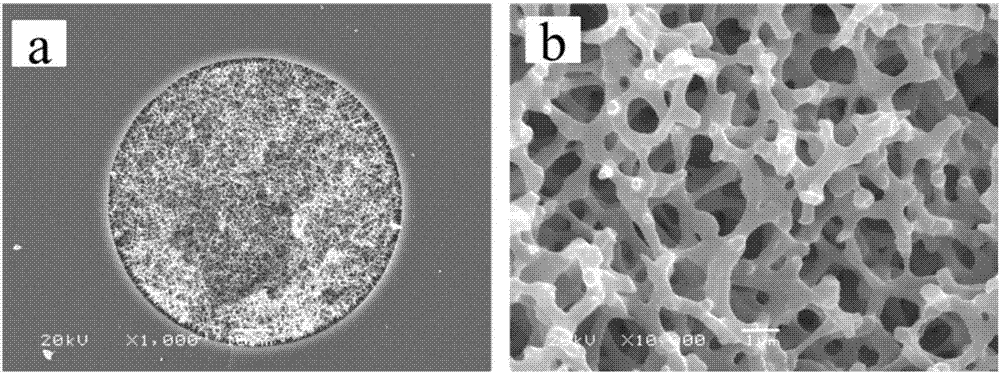
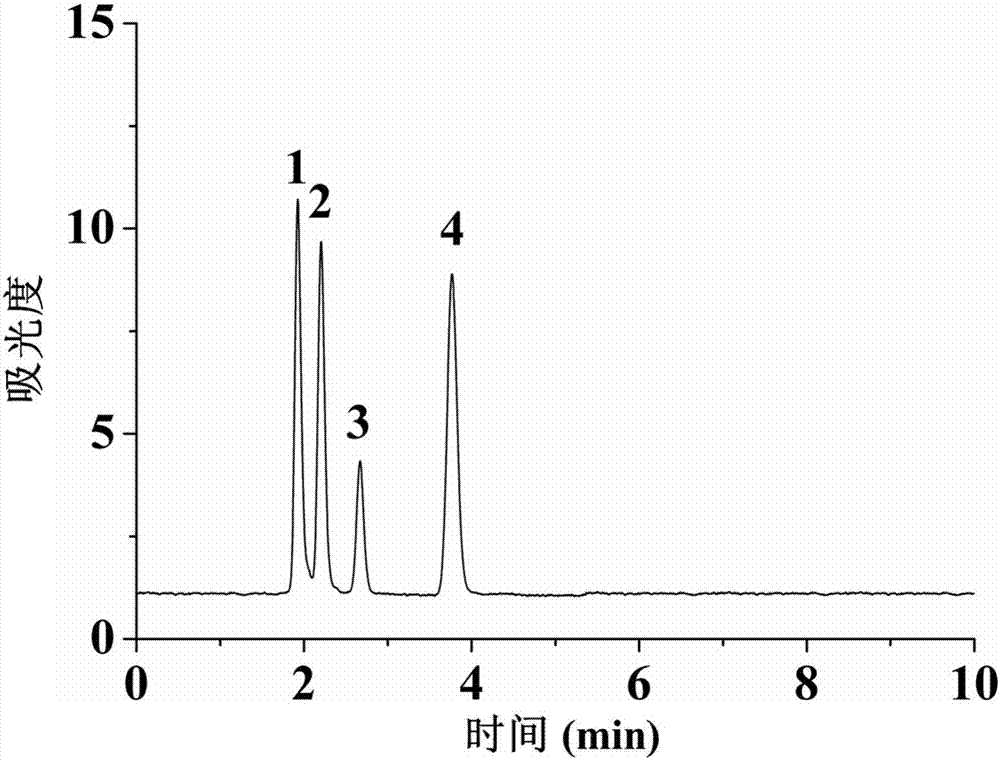
Preparation method and use of organic-inorganic hydrophilic hybrid integral material
An integral material, hydrophilic technology, applied in other chemical processes, chemical instruments and methods, etc., can solve the problems of insufficient preparation repeatability, easy to be affected by pH, etc., and achieve the function modification method is simple, versatile, chromatographic Good separation ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The preparation steps are as follows:
[0023] 1) Add 300mg octavinylsilsesquioxane to the UV transparent centrifuge tube;
[0024] 2) Add 107.9 mg of 1-thioglycerol to the UV transparent centrifuge tube in step 1);
[0025] 3) Add 10mL tetrahydrofuran to the UV transparent centrifuge tube in step 1);
[0026] 4) Add 20 μL of photoinitiator 2,2-dimethoxy-phenylacetophenone (2,2-dimethoxy-2-phenylacetophenone, DMPA) to the UV transparent centrifuge tube in step 1);
[0027] 5) Ultrasonicate the mixed system obtained after step 4) at normal temperature to completely dissolve it to form a uniform transparent solution to remove dissolved oxygen in the mixed system;
[0028] 6) sealing the mixed solution obtained in step 5) in an ultraviolet transparent glass bottle;
[0029] 7) Place the ultraviolet transparent glass bottle sealed with the mixed solution in step 6) under an ultraviolet lamp to react for 20 minutes;
[0030] 8) The product obtained in step 7) the ultravi...
Embodiment 2
[0039] Embodiment 2 preparation steps are as follows:
[0040] 1) Add 300mg octavinylsilsesquioxane to the UV transparent centrifuge tube;
[0041] 2) Add 161.8 mg of 1-thioglycerol to the UV transparent centrifuge tube in step 1);
[0042] 3) Add 10mL tetrahydrofuran to the UV transparent centrifuge tube in step 1);
[0043]4) Add 20 μL of photoinitiator 2,2-dimethoxy-phenylacetophenone (2,2-dimethoxy-2-phenylacetophenone, DMPA) to the UV transparent centrifuge tube in step 1);
[0044] 5) Ultrasonicate the mixed system obtained after step 4) at normal temperature to completely dissolve it to form a uniform transparent solution to remove dissolved oxygen in the mixed system;
[0045] 6) sealing the mixed solution obtained in step 5) in an ultraviolet transparent glass bottle;
[0046] 7) Place the ultraviolet transparent glass bottle sealed with the mixed solution in step 6) under an ultraviolet lamp to react for 20 minutes;
[0047] 8) The product obtained in step 7) the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com