Preparation method for diphenylketone tetraformate serving as light-induced antibacterial finishing agent
A technology of benzophenone tetracarboxylate and benzophenone tetracarboxylic dianhydride is applied in the field of preparation of light-induced antibacterial finishing agent benzophenone tetracarboxylate, which can solve complex production process and harsh reaction conditions , Harmful skin and other problems, to achieve broad-spectrum antibacterial properties, mild reaction conditions, long-lasting antibacterial effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
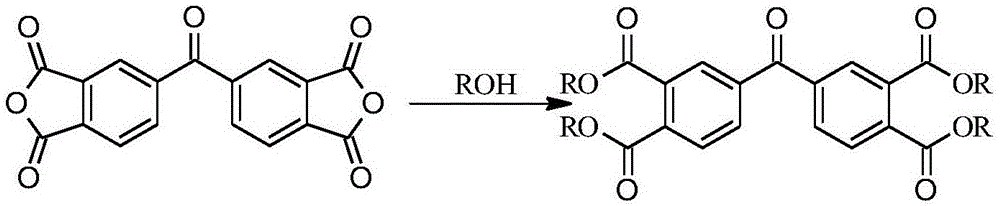
[0037] In a 100mL three-necked flask equipped with a reflux condenser, a water separator and a thermometer, add 10.00g of 3,3',4,4'-benzophenone tetracarboxylic dianhydride and 15.00mL of n-butanol in sequence, 0.27mL of concentrated sulfuric acid was slowly added dropwise, stirred continuously, and the temperature was raised to 120°C for reaction. The reaction was observed to be complete by thin-layer chromatography. After the system was cooled to room temperature, the 2 CO 3 The solution was washed to neutrality, the organic phases were combined, dried with anhydrous sodium sulfate, filtered, and the filtrate was distilled under reduced pressure to obtain crude butyl 3,3',4,4'-benzophenone tetracarboxylate. The mixed solution of dichloromethane and methanol with a ratio of 7:1 was used as an eluent, and column chromatography was carried out for separation and purification to obtain 16.72 g of pure product 3,3',4,4'-benzophenone tetracarboxylate. The rate is 92.45%. The NMR...
Embodiment 2
[0039] In a 100mL three-neck flask equipped with a reflux condenser, a water separator and a thermometer, add 10.00g of 3,3',4,4'-benzophenone tetracarboxylic dianhydride and 8.70mL of ethanol in sequence, slowly Add 0.05mL concentrated sulfuric acid dropwise, stir continuously, heat up to 150°C for reaction, observe the reaction is complete by thin-layer chromatography, wait for the system to cool to room temperature, and use 15% Na 2 CO 3 The solution was washed to neutrality, and the organic phases were combined, dried with anhydrous sodium sulfate, filtered, and the filtrate was distilled under reduced pressure to obtain crude ethyl 3,3',4,4'-benzophenone tetracarboxylate. A mixture of dichloromethane and methanol at a ratio of 7:1 was used as the eluent, and column chromatography was performed for separation and purification to obtain 13.12 g of pure ethyl benzophenone tetracarboxylate with a yield of 90%. The NMR of the product 3,3',4,4'-ethyl benzophenone tetracarboxyl...
Embodiment 3
[0041] The 3,3',4,4'-benzophenone tetracarboxylate obtained in Example 1 is used for finishing polyester fabrics, the specific method is as follows:
[0042] (1) Add 1.5 g of emulsifier Tween 80 to 150 mL of distilled water, stir for 30 min to obtain an emulsifier solution, then slowly add 15 g of 3,3',4,4'-benzophenone tetracarboxylate in Example 1 Esters, stirred for 1h, to obtain a finishing working solution with an effective concentration of antibacterial agent of 100g / L.
[0043](2) Immerse the unfinished polyester fabric in the finishing working solution in step (1), use two dipping and two padding (the liquid-carrying rate is 90%), pre-bake at 80°C for 3min, and bake at 180°C for 2.5min, Get antibacterial polyester fabric. Take the antibacterial polyester fabric, and wash it 30 times with water according to the washing procedure of FZ / T73023-2006 "Antibacterial Knitwear Appendix C Antibacterial Fabric Sample Washing Test Method".
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com