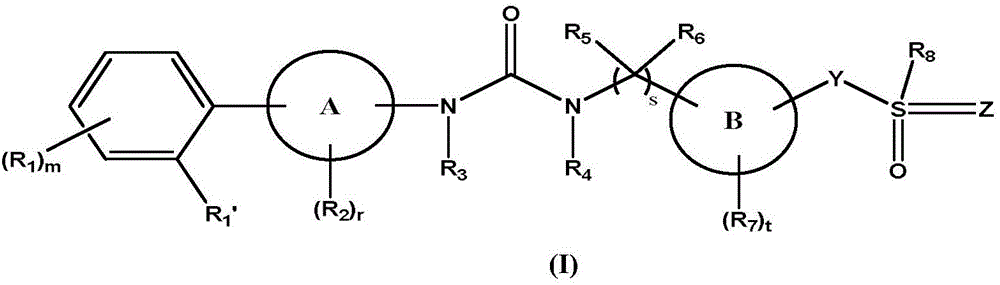
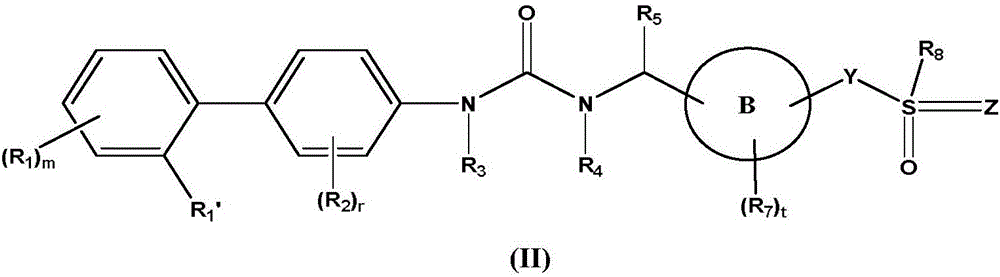
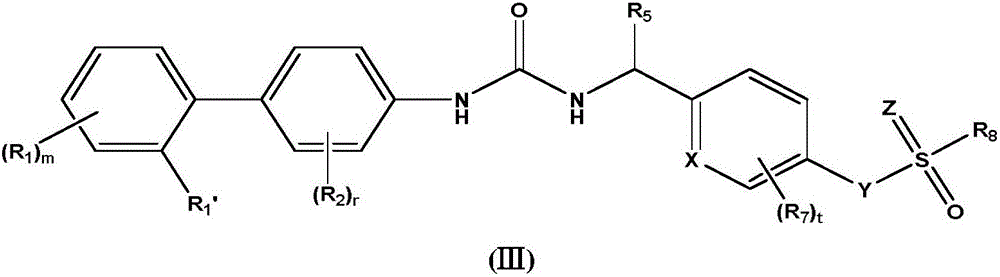
Biaryl urea derivative as well as salt, preparation method and application thereof
A technology of biaryl urea and derivatives is applied in the field of biaryl urea derivatives and their preparation, and can solve the problems of reduced differentiation ability and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0108] Example 1: 1-(2,6-dichloro-2'-(trifluoromethoxy)-[1,1'-biphenyl]-4-yl)-3-(4-(ethylsulfonyl) ) benzyl) urea
[0109] (1-(2,6-dichloro-2'-(trifluoromethoxy)-[1,1'-biphenyl]-4-yl)-3-(4-(ethylsulfonyl)benzyl)urea)
[0110]
[0111] Synthetic Intermediate 1: 2,6-Dichloro-2'-(trifluoromethoxy)-[1,1'-biphenyl]-4-amine
[0112] Step 1: 2-Bromo-1,3-dichloro-5-nitrobenzene
[0113] Add 2,6-dichloro-4-nitroaniline (5g, 24mmol), copper bromide (16g, 72mmol) and acetonitrile (50mL) into the single-necked bottle, and add tert-butyl nitrite (7.46mL) dropwise under stirring in an ice bath. g, 72mmol), then stirred at room temperature and reacted for 6 hours. After the reaction was completed, water (100ml) was added, extracted with ethyl acetate (100mLx2), saturated sodium chloride (100mL), dried over anhydrous sodium sulfate, and concentrated under pressure to obtain an orange solid 6.3 grams, yield 97%;
[0114] Step 2: 4-Bromo-3,5-dichloroaniline
[0115] At room temperature, a...
Embodiment 2
[0125] Example 2: 1-([1,1'-biphenyl]-4-yl)-3-(4-(ethylsulfonyl)benzyl)urea
[0126] (1-([1,1'-biphenyl]-4-yl)-3-(4-(ethylsulfonyl)benzyl)urea)
[0127]
[0128] Add (4-(ethylsulfonyl)phenyl)methanamine (50mg, 0.30mmol), DCM (2mL), and DIEA (77.4mg, 0.6mmol) to a 25mL single-necked bottle and stir in an ice bath for 5 minutes, then add triphosgene ( 26mg, 0.10mmol, add 4-benzidine (60mg, 0.30mmol) after continuing the ice-bath reaction for 30 minutes, continue the ice-bath for 30 minutes, then react overnight at room temperature. Add H 2 O (10mL), extracted with dichloromethane (10mLx3), combined the organic layers, washed the organic layer with saturated sodium chloride (10mL), dried over anhydrous sodium sulfate, filtered, and the filtrate was spin-dried under reduced pressure to obtain a crude product, which was prepared with plate (petroleum ether: ethyl acetate = 1:1) to give white solid 1-([1,1'-biphenyl]-4-yl)-3-(4-(ethylsulfonyl)benzyl)urea 16 mg, yield 22.8%. 1 ...
Embodiment 3
[0129] Example 3: 1-(2-chloro-2'-(trifluoromethoxy)-[1,1'-biphenyl]-4-yl)-3-(4-(ethylsulfonyl)benzyl ) urea
[0130] (1-(2-chloro-2'-(trifluoromethoxy)-[1,1'-biphenyl]-4-yl)-3-(4-(ethylsulfonyl)benzyl)urea)
[0131]
[0132] Step 1: 2-Dichloro-2'-(trifluoromethoxy)-[1,1'-biphenyl]-4-amine
[0133] Add 4-chloro-3-bromoaniline (500mg, 2.43mmol), 2-trifluoromethoxyphenylboronic acid (649mg, 3.15mmol), Pd 2 (dppf)Cl 2 (69mg, 0.12mmol), potassium carbonate (1.01g, 7.29mmol), acetonitrile / water (4ml / 1ml). Nitrogen gas was bubbled for 5 minutes, and then microwaved at 100°C for 2 hours. Wash with saturated ammonium chloride (20ml). Silica gel column separation (eluent petroleum ether: ethyl acetate = 10:1-5:1) to obtain yellow oil 2-dichloro-2'-(trifluoromethoxy)-[1,1'-biphenyl Base]-4-amine 610mg, yield 88.5%. 1 H NMR (400MHz, CDCl 3 )δ7.42–7.35(m,1H),7.36–7.28(m,3H),7.06-7.04(d,J=8.2Hz,1H),6.81-6.80(d,J=1.9Hz,1H),6.64 -6.62(d,J=8.2Hz,1H),3.57(s,2H).MS(ESII)m / z:288.0(MH+)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com