Chronic respiratory disease therapeutic agent and cardiac fibrillation suppressing composition
A technology for respiratory diseases and cardiac fibrosis, applied in respiratory diseases, cardiovascular diseases, drug combinations, etc., can solve the problems of induced myocardial damage, dosage dependence, etc., and achieve improvement of morbidity, inhibition of pulmonary or cardiac fibrosis , excellent pharmacological activity and biological adaptability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
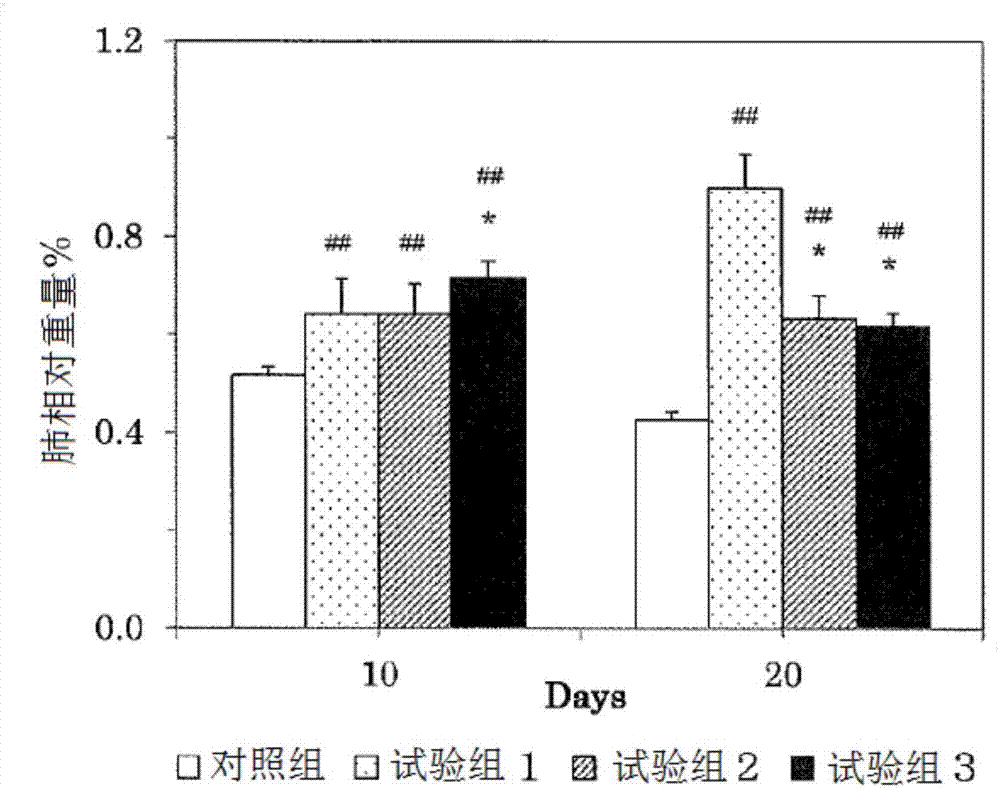
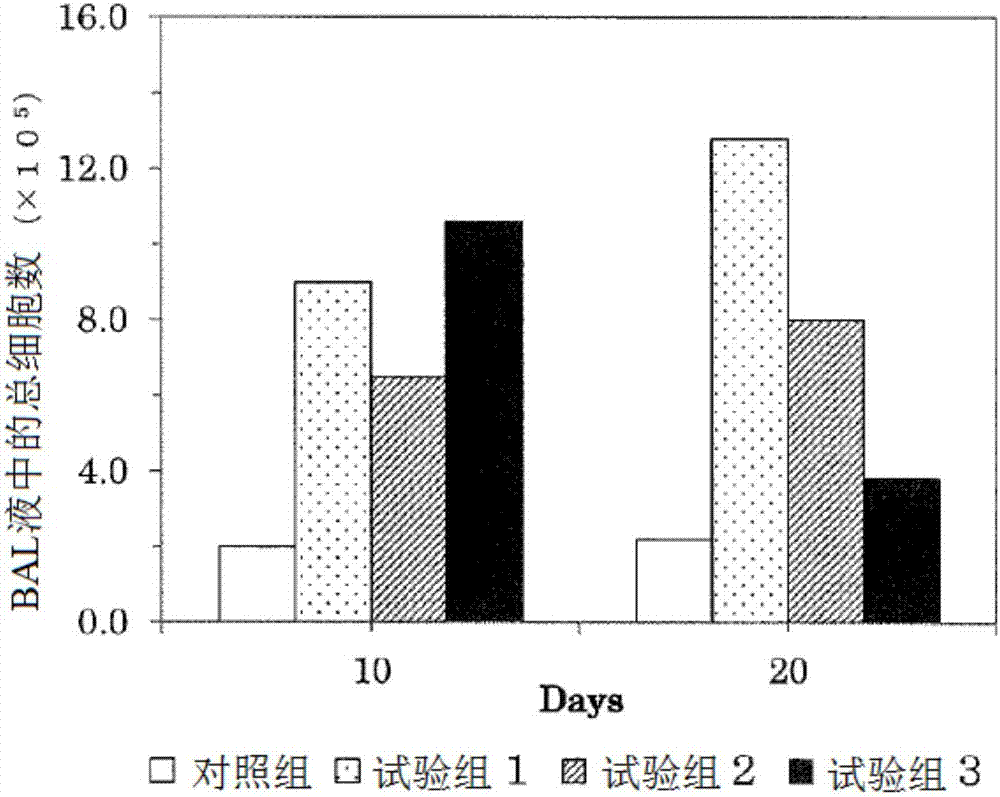
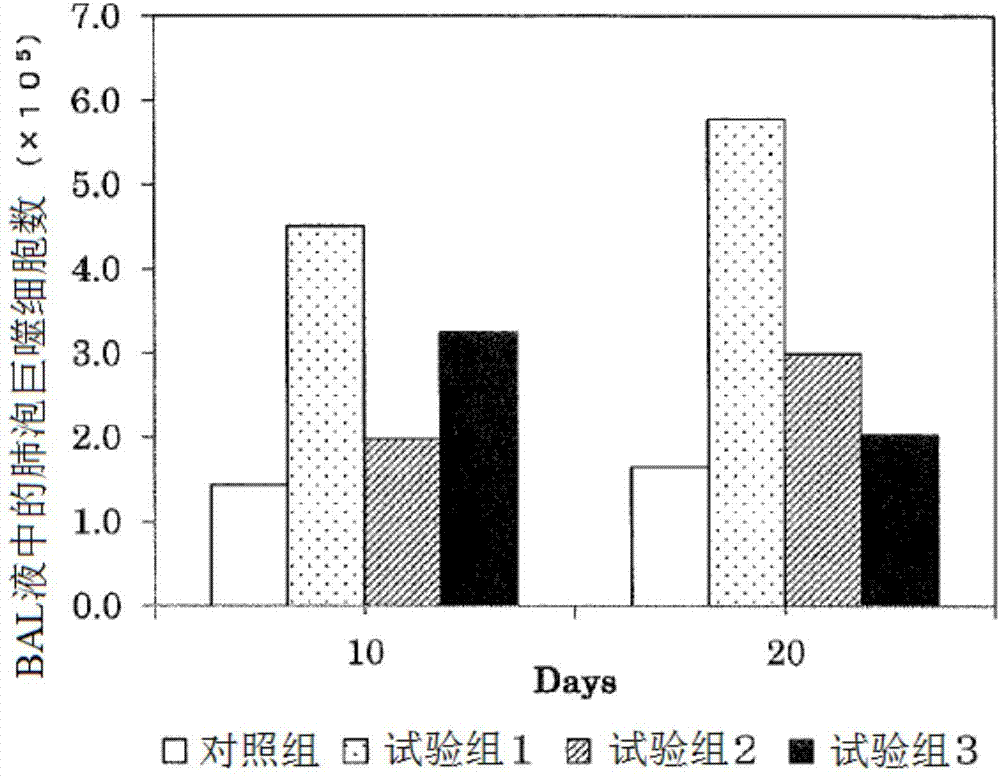
[0082] 1. Study on the effect on pulmonary fibrosis induced by bleomycin
[0083] Bleomycin is used to make disease model animals of interstitial pneumonia. The bleomycin was administered to male SD rats aged 10 weeks after birth, and the 2,3,5-hydroquinone derivative represented by the above general formula (1) of the present invention was administered. The effects of trimethylhydroquinone-1-hexyl ether (HTHQ) were investigated. The composition of the test group is as follows: control group: administration of sterilized normal saline, test group 1: single administration of bleomycin (7.5mg / kg body weight), test group 2: combined administration of bleomycin (7.5mg / kg body weight). kg body weight) and HTHQ (50 mg / kg body weight / day), test group 3: combined administration of bleomycin (7.5 mg / kg body weight) and HTHQ (200 mg / kg body weight / day).
[0084] Regarding the test groups 1 to 3, the bleomycin was administered once, and the administration was performed orally. In addi...
Embodiment 2
[0101] 2. Research on the effect on lung inflammation induced by tobacco smoke
[0102] Six-week-old SPF C57BL / 6N male mice weighing 20 to 25 g were purchased from Coretec Co., Ltd. (Korea). After a quarantine and acclimatization period of about 1 week, the mice were divided into 5 groups as shown in Table 2 below.
[0103] [Table 2]
[0104]
[0105] The test was carried out as follows. In the test group, the COPD (chronic obstructive pulmonary disease) model group was exposed to tobacco smoke (8 cigarettes / day) for 1 hour for 10 days, and the intranasal LPS was administered (5 µg / 50 µL / mouse). For the positive control substance administration group, 10 mg / kg body weight / day of roflumilast as a positive control substance was orally administered for 10 days, followed by exposure to tobacco smoke for 1 hour after 1 hour. In addition, LPS (5 µg / 50 µL / mouse) was intranasally administered on the 8th day from the start of the test. Here, roflumilast is a selective phosphodi...
Embodiment 3
[0114] 3. Research on the effect on asthma
[0115] The effectiveness of the hydroquinone derivative represented by the above general formula (1) of the present invention on asthma was investigated using an allergic asthma model in mice sensitized with ovalbumin.
[0116] 6-week-old BALB / c female mice were purchased, and after about 2 weeks of acclimatization and feeding, the mice were allocated 5 mice in each of the 5 groups shown in Table 3 below.
[0117] [table 3]
[0118]
[0119] test to Figure 12 The indicated sensitization, elicitor and test substance dosing schedules were performed. Figure 12 "IP" in "IP" indicates ovalbumin sensitization treatment by intraperitoneal administration of ovalbumin / aluminum hydroxide, "IH" indicates inhalation exposure treatment of ovalbumin, and "PO" indicates administration of each test substance. Specifically, 200 μL of PBS (pH 7.4) emulsified by adding 20 μg of ovalbumin and 2 mg of aluminum hydroxide as an adjuvant was intrap...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com