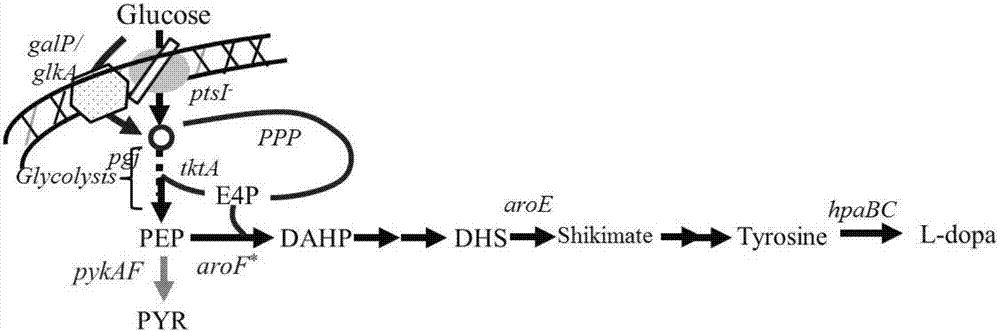
Escherichia coli recombination strain for producing levodopa as well as construction method and application thereof
A technology of recombinant strains and levodopa, which is applied in the biological field and can solve problems such as not being able to meet market demand and urgently increasing the production of levodopa
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0124] The construction of embodiment 1 escherichia coli recombinant strain T002
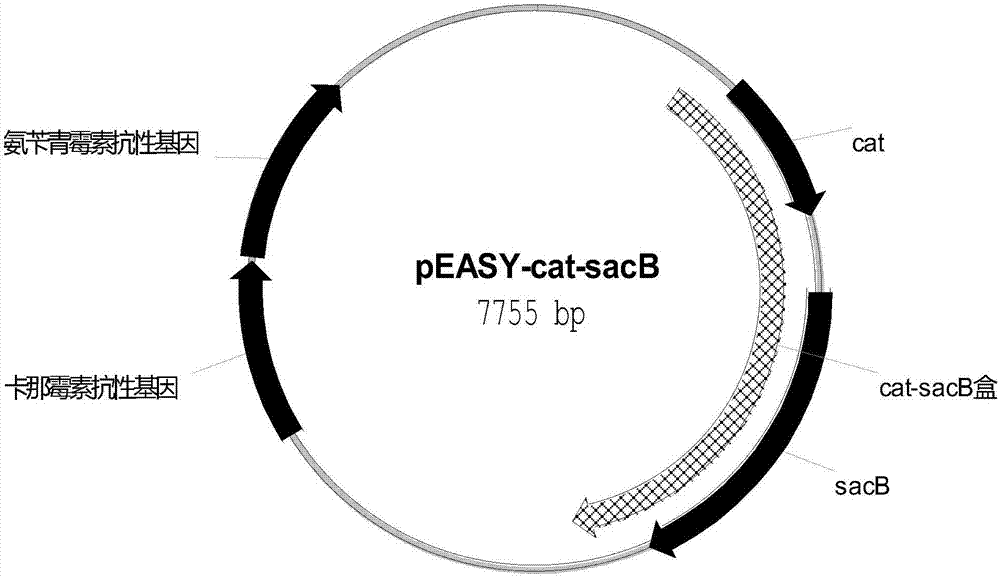
[0125] With the plasmid pEASY-cat-sacB (as shown in SEQ ID NO:5) containing chloramphenicol resistance gene cat and fructan sucrose transferase gene sacB figure 2 (shown) is a template, and primer 21 aroE1-up / aroE1-down is used to amplify the fragment aroE1 of homologous recombination in the first step. The sequence of primer 21 is:
[0126] aroE1-up (forward primer):
[0127] GATGCCCTGACGGGTGAACTGTTTCGACAGGGGTAACATA GTGACGGAAGATCACTTC
[0128] aroE1-down (reverse primer):
[0129] CTGTGGGCTATCGGATTACCAAAAACAGCATAGGTTTCCA ATCAAAGGGAAAACTGTCC
[0130] Amplification system: 5×TransStart TM FastPfu Buffer 10μL, dNTPs (2.5mmol / L each dNTP) 4μL, DNA template 1μL (20-50ng), forward primer (10μmol / L) 2μL, reverse primer (10μmol / L) 2μL, 100% DMSO 1μL, TransStart TM FastPfu DNA Polymerase (2.5U / μL) 1 μL, deionized water 29 μL, total volume 50 μL.
[0131] The amplification conditions are: 94°...
Embodiment 2
[0151] The construction of embodiment 2 escherichia coli recombinant strain T004
[0152] With the plasmid pEASY-cat-sacB (as shown in SEQ ID NO:5) containing chloramphenicol resistance gene cat and fructan sucrose transferase gene sacB figure 2 (shown) is used as template, and primer t41 hpaBC-up / hpaBC-down is used to amplify the fragment hpaBC1 of homologous recombination in the first step. The primer t41 sequence is:
[0153] hpaBC-up (forward primer):
[0154] AACTATGAACATTGTCGATCAACAAACTTTTCGCGATGCG GTGACGGAAGATCACTTC
[0155] hpaBC-down (reverse primer):
[0156] TCCGTGGTGATGATATTGACCGCCGCGCCCATGCAGGACA ATCAAAGGGAAAACTGTCC
[0157] Amplification system: 5×TransStart TM FastPfu Buffer 10μL, dNTPs (2.5mmol / L each dNTP) 4μL, DNA template 1μL (20-50ng), forward primer (10μmol / L) 2μL, reverse primer (10μmol / L) 2μL, 100% DMSO 1μL, TransStart TM FastPfu DNA Polymerase (2.5U / μL) 1 μL, deionized water 29 μL, total volume 50 μL.
[0158] The amplification conditions...
Embodiment 3
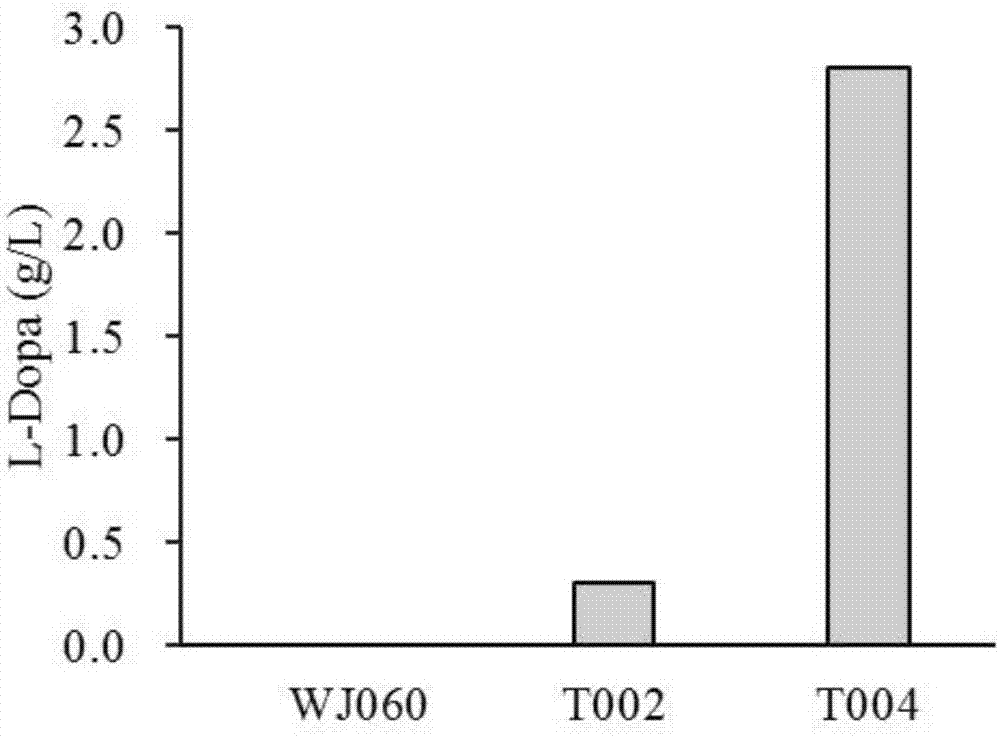
[0180] Example 3 Escherichia coli recombinant strain WJ060, recombinant strain T002 and recombinant strain T004 fermented to produce levodopa
[0181] The various components in the seed medium or fermentation medium mentioned in the present invention and their content, fermentation temperature, pH value of the fermentation system, fermentation time, and inoculum size can be adjusted accordingly according to needs. E.g:
[0182] The initial glucose content is 20g / L-100g / L, specifically 20g / L or 30g / L or 40g / L or 50g / L or 60g / L or 70g / L or 80g / L or 90g / L or 100g / L etc. (after the start of fermentation, when the glucose concentration in the fermenter drops below 1g / L, start feeding with a glucose solution with a concentration of 500g / L-600g / L, and control the feeding speed so that the glucose concentration in the fermenter less than 1g / L);
[0183] The content of yeast extract is 0-5g / L, specifically 0g / L or 1g / L or 2g / L or 3g / L or 4g / L or 5g / L;
[0184] The content of trypto...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com