Method for modifying protein by using transpeptidase Sortase A
A technology of transpeptidase and protein, applied in the field of genetic engineering, can solve the problems of low expression level, difficult product purification, high cost, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1 Preparation of Sortase A extracellular expression crude enzyme solution
[0022] Using pET22a as the expression vector, and using the PelB signal peptide on the vector to construct a recombinant expression vector carrying the gene encoding Sortase A (SEQ ID NO.1), the recombinant expression vector and the molecular chaperone pG-Tf2 plasmid were co-transformed into the large intestine Bacillus E. coli BL21 (DE3). With TB medium (peptone 12g / L, yeast powder 24g / L, glycerol 4g / L, KH 2 PO 4 23.1g / L, K 2 HPO 4 125.4g / L) is the fermentation medium, 50mL of liquid in 500mL shake flask, fermentation temperature 30°C, inoculum size 2%, induction time OD 600 =0.6, induction temperature 30°C, inducer IPTG concentration 100mM / L, induction fermentation time 36h. The obtained fermentation broth was centrifuged (12000rpm for 5 minutes) to remove the bacterium, and 90.2 mg / L Sortase A crude extracellular enzyme liquid could be obtained.
Embodiment 2
[0023] Example 2 Effects of Sortase A Crude Enzyme Liquid and His Tag-labeled Affinity Nickel Magnetic Beads Binding Conditions on Sortase A Enzyme Activity
[0024] The effects of the amount of Sortase A crude enzyme solution and magnetic beads, combined temperature, time and pH on the enzyme activity of magnetic beads are shown in Table 1-Table 3. It can be seen that on the basis of repeated use of magnetic beads 5 times, in order to ensure that the Sortase The catalytic effect of a common dose (10μM) requires the use of more than 3mL of Sortase A crude enzyme solution (90.2mg / L); the combined temperature, time and pH value have a significant impact on the catalytic effect of the enzyme, the pH of the crude enzyme solution When the value is 5.0-8.0, the effect of binding at 4-25°C for more than 30min is better; in addition, in order to save the cost of using magnetic beads, the enzyme activity per unit volume of magnetic beads was tested, and it was determined that the enzyme...
Embodiment 3
[0025] Example 3 Prepare a large number of Sortase A-bound magnetic beads, and detect the properties of the obtained magnetic beads
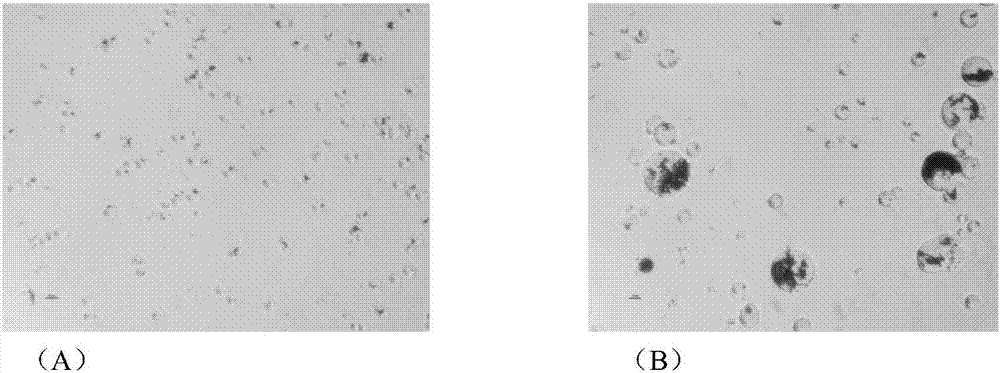
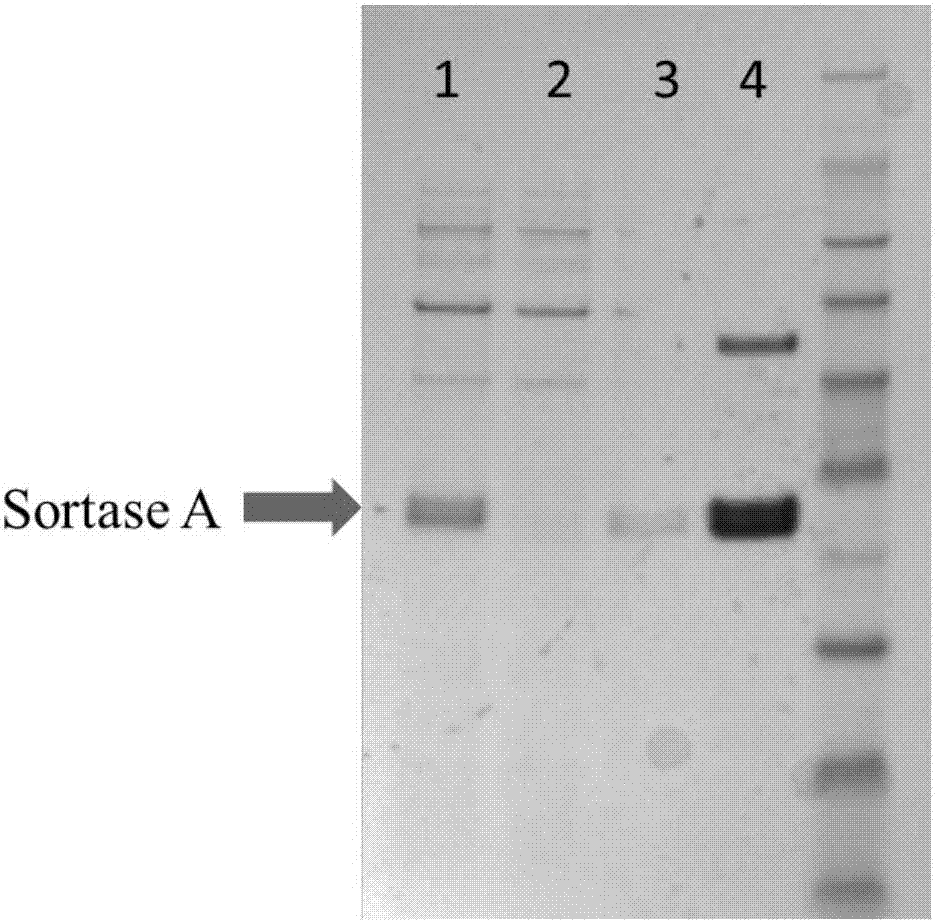
[0026]Based on the optimized binding conditions, a small amount of Sortase A-bound magnetic beads (200 μL) with activity equivalent to that of 10 μM free enzyme was successfully prepared, and on this basis, the preparation system was scaled up (10 mL). The enzymatic activity of the magnetic beads prepared by the scale-up system can reach 318.2U / 200μL magnetic beads; the microscopic examination shows that the average particle diameter increases from 19 μm to 34 μm after the magnetic beads are combined with Sortase A ( figure 1 ); As detected by SDS-Page, Sortase A in the crude enzyme solution can be completely combined after the magnetic beads are combined with Sortase A, and after elution with imidazole, it can be proved that the magnetic beads only bind the C-terminus with His 6 Labeled Sortase A( figure 2 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com