Preparation method and application of catalyst for NOx removal
A nitrogen oxide and catalyst technology, applied in the field of catalysts and their preparation, can solve problems such as easy deactivation, and achieve the effects of good resistance, high purification rate, and cheap and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
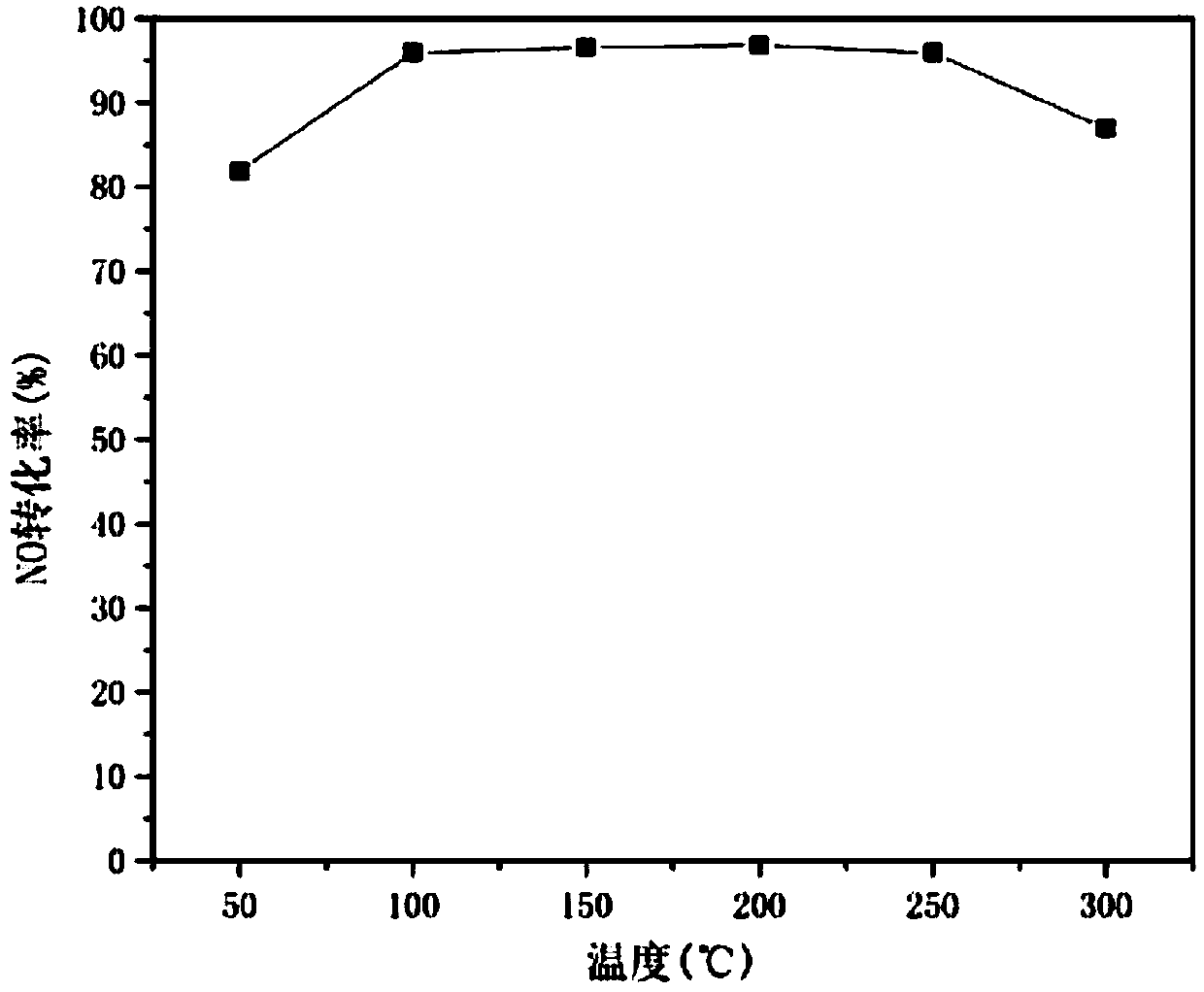
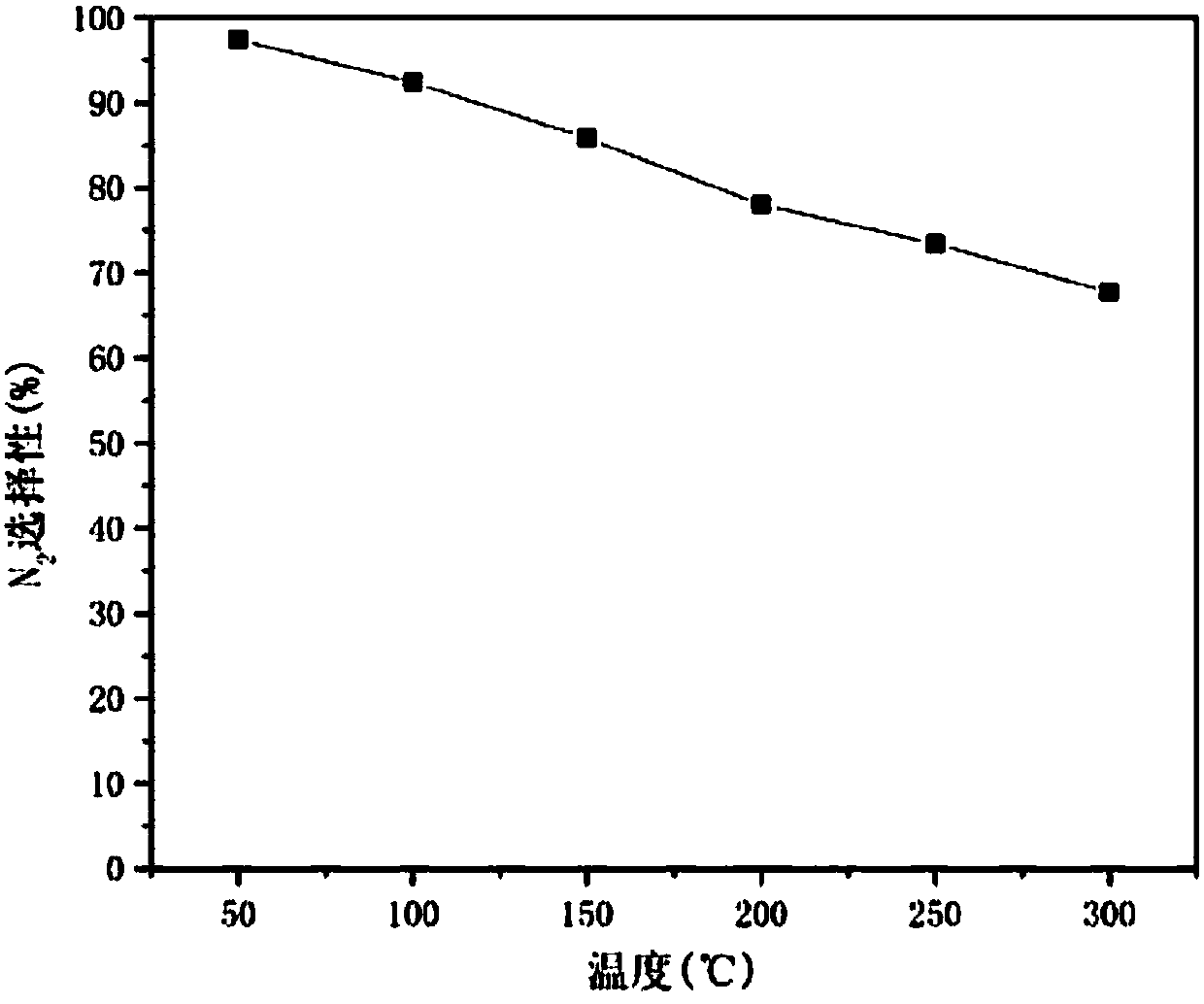
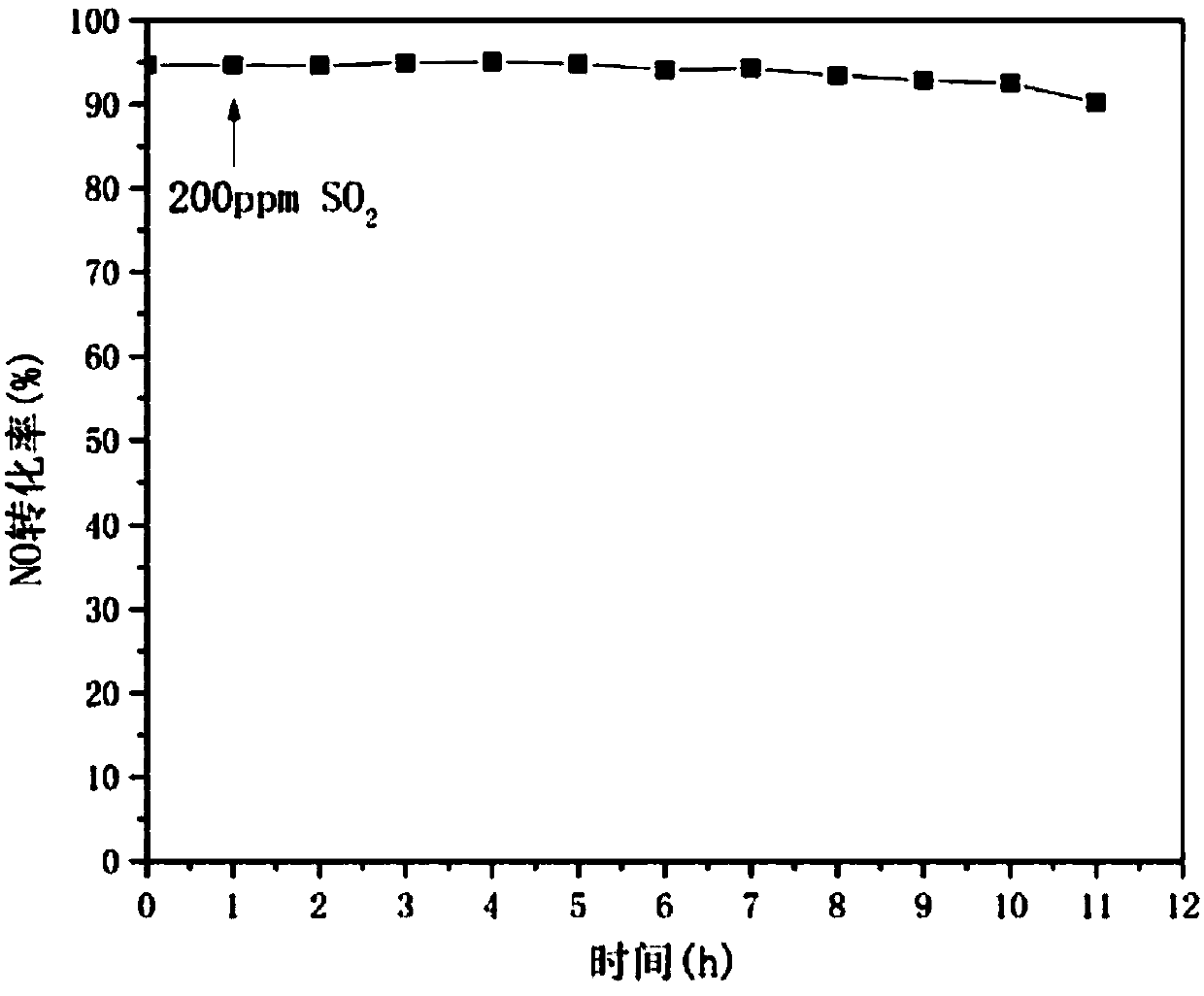
Examples
Embodiment 1
[0023] Embodiment 1, prepare a kind of catalyst that removes nitrogen oxide, the steps are as follows:
[0024] Step 1, take 0.025mol Mn(NO 3 ) 2 ·6H 2 O and 0.005mol Ni(NO 3 ) 2 ·6H 2 O was dissolved in distilled water and configured into solution A1, so that the molar ratio of Ni to Mn was 0.2:1. Take 0.0025molCe(NO 3 ) 2 ·6H 2 O was dissolved in distilled water so that the molar ratio of Ce to Mn in solution A1 was 0.1:1 to prepare solution B1. Take 0.125mol Na 2 CO 3 Dissolve in 250ml distilled water to make solution C1.
[0025] Step 2. Drop the above-prepared solution C1 into the flask containing solution A1 drop by drop, and stir vigorously at the same time. By adjusting the drop rate of solution C1 to control the pH of the titration end point of the mixed solution = 8, continue to stir for 20 minutes, and keep at room temperature. Aging for 10 hours and filtering to obtain filter cake A1; drip solution C1 dropwise into the flask containing solution B1 while...
Embodiment 2
[0029] Embodiment 2, prepare a kind of catalyst that removes nitrogen oxide, the steps are as follows:
[0030]Step 1, take 0.025mol Mn(NO 3 ) 2 ·6H 2 O and 0.01mol Ni(NO 3 ) 2 ·6H 2 O was dissolved in distilled water and configured as solution A2, so that the molar ratio of Ni to Mn was 0.4:1. Take 0.005molCe(NO 3 ) 2 ·6H 2 O was dissolved in distilled water so that the molar ratio of Ce to Mn in solution A2 was 0.2:1 to prepare solution B2. Take 0.125mol Na 2 CO 3 Dissolve in 250ml distilled water to make solution C2.
[0031] Step 2. Drop the above-prepared solution C2 into the flask containing solution A2 drop by drop, and stir vigorously at the same time. By adjusting the drop rate of solution C2 to control the pH of the titration end point of the mixed solution = 9, continue to stir for 30 minutes, and keep at room temperature. Aging for 12 hours, filtering to obtain filter cake A2; drop solution C2 into the flask containing solution B2 drop by drop, while st...
Embodiment 3
[0035] Embodiment 3, prepare a kind of catalyst that removes nitrogen oxide, the steps are as follows:
[0036] Step 1, take 0.025mol Mn(NO 3 ) 2 ·6H 2 O and 0.015mol Ni(NO 3 ) 2 ·6H 2 O was dissolved in distilled water and configured as solution A3, so that the molar ratio of Ni to Mn was 0.6:1. Take 0.0075molCe(NO 3 ) 2 ·6H 2 O was dissolved in distilled water so that the molar ratio of Ce to Mn in solution A3 was 0.3:1 to prepare solution B3. Take 0.125mol Na 2 CO 3 Dissolve in 250ml distilled water to make solution C3.
[0037] Step 2. Drop the above-prepared solution C3 into the flask containing solution A3 drop by drop, and stir vigorously at the same time. By adjusting the drop rate of solution C3 to control the pH of the titration end point of the mixed solution to 10, continue to stir for 50 minutes, and keep at room temperature. Aging for 14 hours and filtering to obtain filter cake A3; drop solution C3 into the flask containing solution B3 drop by drop, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com