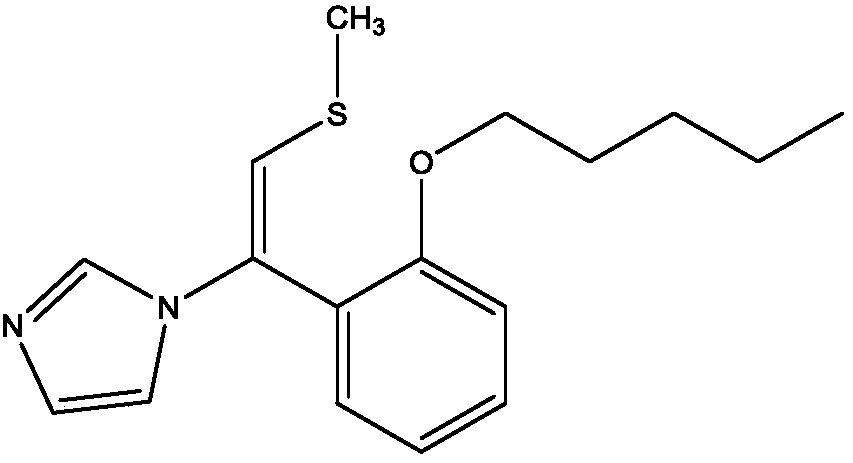
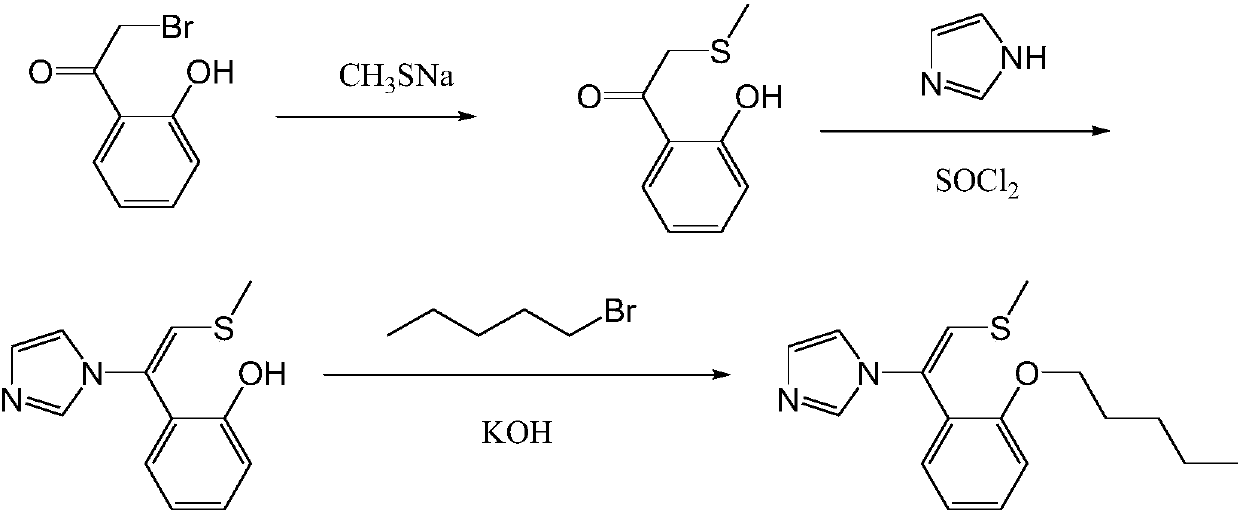
Neticonazole hydrochloride preparation method
A technology of neconazole hydrochloride and imidazole, which is applied in the field of preparation of neconazole hydrochloride, can solve the problems of high operation risk and non-environmental protection, and achieve the effects of simple operation and post-treatment, content control, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
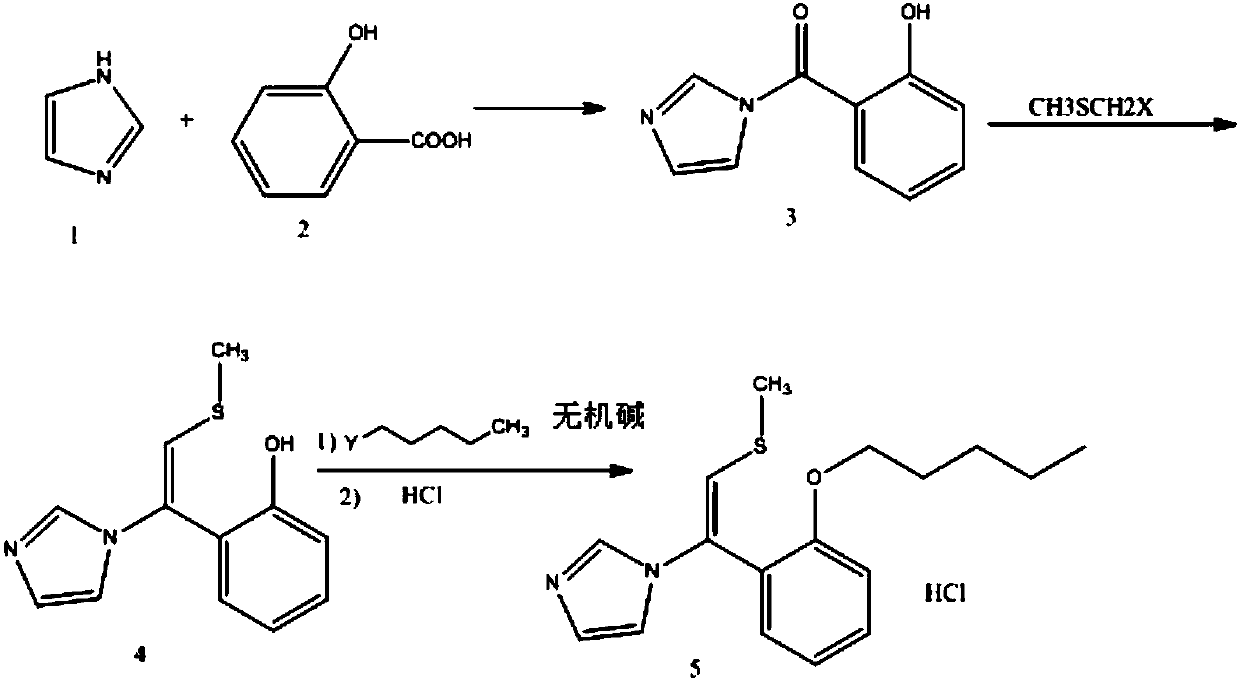
[0029] Embodiment 1: the preparation of formula (3) compound 1-[1-(2-hydroxyphenyl)-2-methanone]-1H imidazole
[0030] Dissolve 30.36g (0.22mol) o-hydroxybenzoic acid in 100mL DMF, add 26.86g (0.2mol) HOBt, 40.95g (0.2mol) DCC in turn, stir at room temperature (about 25-30°C) for 1 hour, then add 13.6g (0.2mol) imidazole, continue to stir at room temperature (at about 25-30°C) for 4 hours, pour the reaction solution into 200mL water, extract twice with 150mL dichloromethane, then successively wash with 5% hydrochloric acid, 1M sodium bicarbonate solution to wash the organic phase. Concentrate the solvent under reduced pressure to obtain a yellow oil, add 80 mL of ethanol, heat to reflux to dissolve the oil, cool down and crystallize for 1 hour, filter, and wash with a small amount of ethanol to obtain 34.55 g of off-white solid product, yield: 91.9%, HPLC purity ( Area normalization method): 98.45%.
Embodiment 2
[0031] Embodiment 2: the preparation of formula (3) compound 1-[1-(2-hydroxyphenyl)-2-methanone]-1H imidazole
[0032] Dissolve 30.36g (0.22mol) o-hydroxybenzoic acid in 100mL DMF, add 26.86g (0.2mol) HOBt, 40.95g (0.2mol) DCC in turn, stir at room temperature (about 25-30°C) for 1 hour, then add 13.6g (0.2mol) imidazole, cooled to 0-10°C and stirred for 5 hours, poured the reaction solution into 200mL water, extracted twice with 150mL dichloromethane, then washed the organic phase with 5% hydrochloric acid and 1M sodium bicarbonate solution successively . Concentrate the solvent under reduced pressure to obtain a yellow oil, add 80 mL of ethanol, heat to reflux to dissolve the oil, cool down and crystallize for 1 hour, filter, and wash with a small amount of ethanol to obtain 31.28 g of off-white solid product, yield: 83.2%, HPLC purity ( Area normalization method): 98.57%.
Embodiment 3
[0033] Embodiment 3: the preparation of formula (3) compound 1-[1-(2-hydroxyphenyl)-2-methanone]-1H imidazole
[0034] Dissolve 30.36g (0.22mol) o-hydroxybenzoic acid in 100mL DMF, add 26.86g (0.2mol) HOBt, 40.95g (0.2mol) DCC in turn, stir at room temperature (about 25-30°C) for 1 hour, then add 13.6g (0.2mol) imidazole, heated to 45-50°C and stirred for 3 hours, poured the reaction liquid into 200mL water, extracted twice with 150mL dichloromethane, then washed the organic phase with 5% hydrochloric acid and 1M sodium bicarbonate solution successively . The solvent was concentrated under reduced pressure to obtain a dark brown oil. Add 80mL of ethanol, heat to reflux to dissolve the oil, cool down and crystallize for 1 hour, filter, wash with a small amount of ethanol to obtain 34.07g of off-white solid product, yield: 90.6%, HPLC purity (area normalization method): 96.53%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com