Mutant of glutamine transaminase expressed by active form
A technology of glutamine and transaminase, applied in the field of enzyme engineering, can solve the problems of low production of transglutaminase and increase of production of transglutaminase, and achieve the effects of easy separation and purification, strong secretion ability and wide pH adaptability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The construction of embodiment 1 recombinant bacteria po1h / hpro-mTG
[0040] The plasmid pINA1297 / N355Q reserved in the laboratory was used as a template, and P1 and P2 were used as primers to carry out PCR, and the 1297 expression vector containing the hpro zymogen region was amplified by PCR. The PCR amplification system is: template 1 μL, upstream and downstream primers 1 μL, primeSTAR 25 μL, double distilled water 22 μL. The PCR conditions are: 98°C for 3min, 98°C for 10s, 60°C for 5s, 72°C for 5min30s, 72°C for 20min, 30 cycles. The plasmid pET 20b / mpro-mTG reserved in the laboratory was used as a template, and P3 and P4 were used as primers to carry out PCR, and the gene fragment containing mTG was amplified by PCR. The PCR amplification system is the same as above, and the PCR conditions are: 98°C for 3min, 98°C for 10s, 60°C for 5s, 72°C for 1min 20s, 72°C for 10min, 30 cycles. The two PCR products were digested by Dpn I and recovered from the gel. The recover...
Embodiment 2
[0043] The construction of embodiment 2 recombinant bacteria po1h / mpro-mTG
[0044] Plasmid pINA1297 / N355Q was digested with fast cutting enzymes Sfi I and BamH I, and then gel recovered to obtain the linearized pINA1297 gene fragment. The plasmid pET 20b / mpro-mTG reserved in the laboratory was used as a template, and P5 and P4 were used as primers to carry out PCR, and the gene fragment of mpro-mTG was amplified by PCR. The PCR amplification system was the same as in Example 1, and the PCR conditions were: 98°C for 3min, 98°C for 10s, 60°C for 5s, 72°C for 1min 20s, 72°C for 10min, 30 cycles. The PCR product was digested by Dpn I and then gel-recovered to obtain the gene fragment of mpro-mTG. The pINA1297 gene fragment and the mpro-mTG gene fragment were mixed at a molar ratio of 1:2, connected using the One Step Cloning Kit, transformed into E.coli JM109, and positive transformants were screened by colony PCR. Pick out 2 positive transformants and inoculate them into LB li...
Embodiment 3
[0045] Embodiment 3 Recombinant bacteria po1h / hpro-mTG and recombinant bacteria po1h / mpro-mTG shake flask fermentation
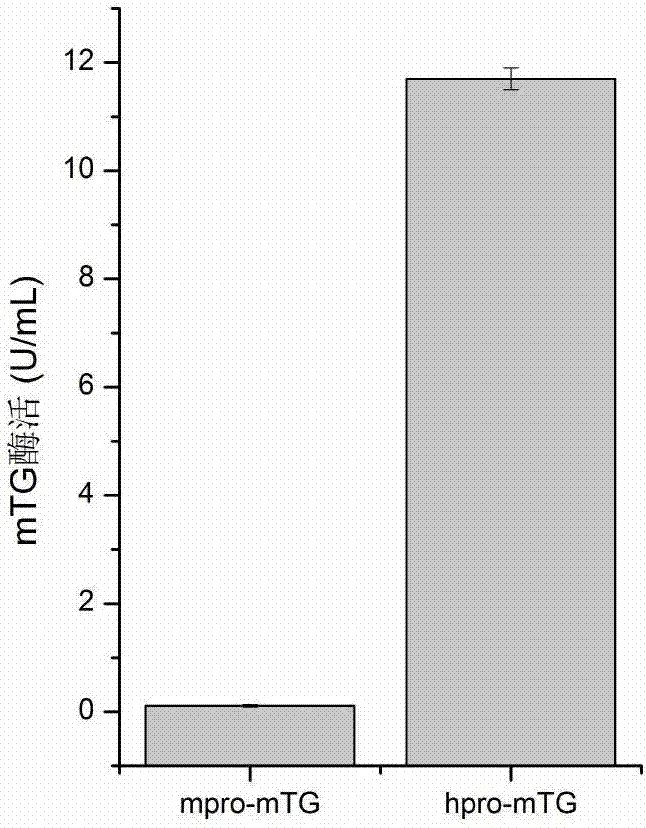
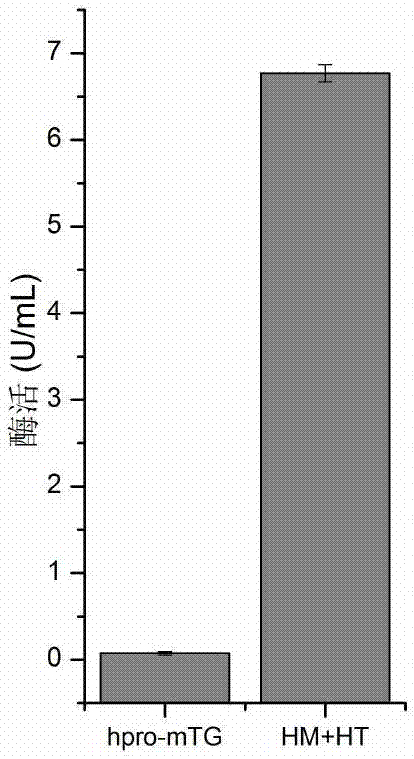
[0046] The recombinant bacterium hpro-mTG constructed in Example 1 and the recombinant bacterium po1h / mpro-mTG constructed in Example 2 were respectively inoculated in YPD liquid medium, and after culturing for 24 hours at 28° C. at 200 rpm, transfer was performed at a 10% inoculum size. Cultivate in Yarrowia lipolytica fermentation medium at 28°C, 200rpm shake flask (specification: 250mL) for 120h. The fermentation broth was centrifuged at 4°C and 4000rpm for 10 minutes, and the supernatant was the crude extracellular enzyme solution. After being activated by dispase, the enzyme activity was measured. The enzyme activities were found to be 11.7U / mL and 0.11U / mL respectively. In the recombinant strain po1h / hpro-mTG, the enzyme activity was increased by 106 times compared with the control after the zymogen region was replaced by hpro ( figure 1 ). The resu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com