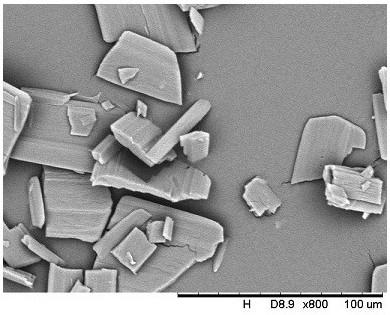
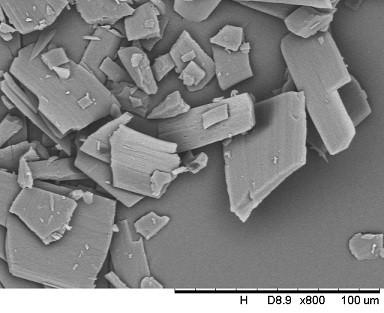
A kind of crystallization method of cephalosporin antibiotic
A technology of antibiotics and cephalosporins, which is applied in the field of industrial production process of cephalosporins antibiotics, can solve the problems of unfavorable quality and efficiency of downstream preparations, uneven distribution of crystal particle size, unsatisfactory crystal shape, etc., and improve equipment utilization rate and fluidity Good, good crystal integrity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1 is substantially the same as the steps of the above-mentioned specific examples, and different process parameters and conditions are as follows:
[0044] A crystallization method of cephalexin, the crystallization process is carried out continuously in 2 crystallization kettles, the crystallization reaction is first carried out in the primary crystallization kettle, and then introduced into the secondary crystallization kettle, the volume ratio of the primary crystallization kettle to the secondary crystallization kettle It is 1:2.
[0045] Specific steps are as follows:
[0046] A. Add water to the primary crystallization kettle, keep stirring and control the crystallization temperature at 25°C, add seed crystals, the mass ratio of water and seed crystals is 8:1, and add raw materials and ammonia water at the same time, so that the pH is controlled at 2.9; The raw material is obtained by dissolving the enzyme with 15% dilute sulfuric acid after separating...
Embodiment 2
[0050] The steps of embodiment 2 are substantially the same as embodiment 1, and different process parameters and conditions are as follows:
[0051] A crystallization method of cephalexin, the crystallization process is carried out continuously in two crystallization kettles, and the volume ratio of the primary crystallization kettle and the secondary crystallization kettle is 1:5.
[0052] Specific steps are as follows:
[0053] A. The crystallization temperature in the primary crystallization kettle is at 45°C, and the pH is controlled at 4.0; the mass ratio of adding water and seed crystals is 80:1, and the raw material is cephalosporin crude product slurry separation enzyme, and then use 20% dilute sulfuric acid Dissolved, the concentration is 200mg / ml. The flow rate of feedstock was 4000 L / h.
[0054] When the volume of the feed liquid in the primary crystallization kettle reaches 4 / 5 of the total volume, start to introduce the feed liquid in the primary crystallizatio...
Embodiment 3
[0057] The steps of embodiment 3 are substantially the same as embodiment 1, and different process parameters and conditions are as follows:
[0058] A crystallization method of cephalexin, the crystallization process is carried out continuously in two crystallization kettles, the volume ratio of the primary crystallization kettle and the secondary crystallization kettle is 1:3.
[0059] Specific steps are as follows:
[0060] A. The crystallization temperature in the primary crystallization kettle is at 35°C, and the pH is controlled at 3.5; the mass ratio of adding water and seed crystals is 50:1, and the raw material is 18% dilute sulfuric acid after the cephalosporin crude product slurry is separated from the enzyme Dissolved, the concentration is 140mg / ml. The flow rate of feedstock was 2500 L / h.
[0061] When the volume of the feed liquid in the primary crystallization tank reaches 70% of the total volume, start to import the feed liquid in the primary crystallization ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com