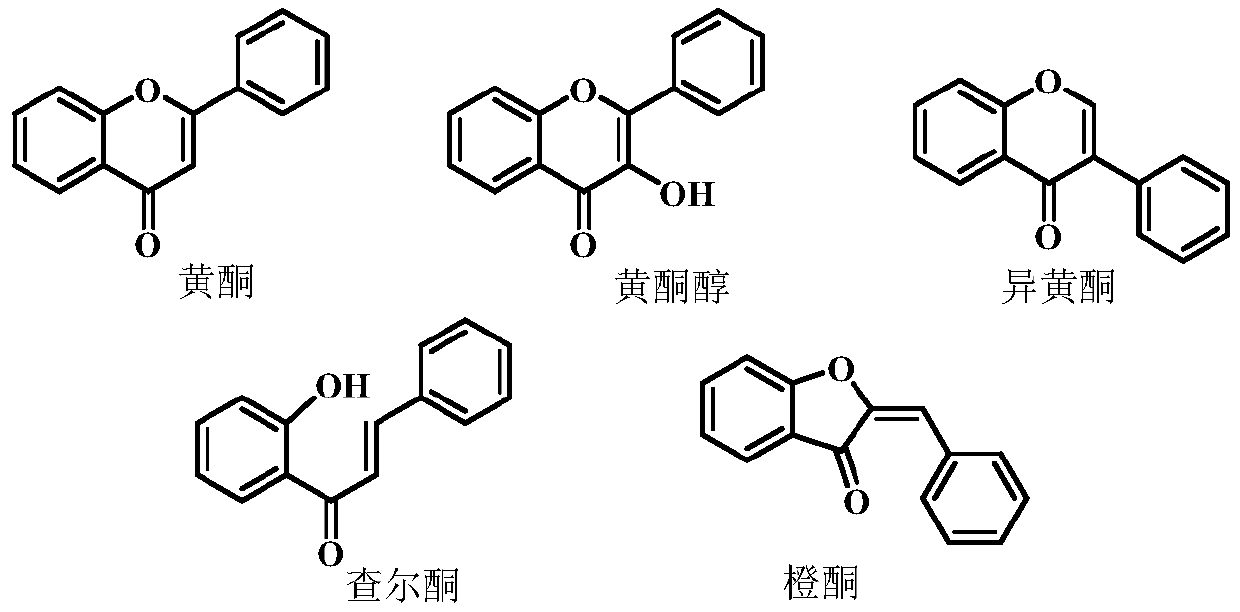
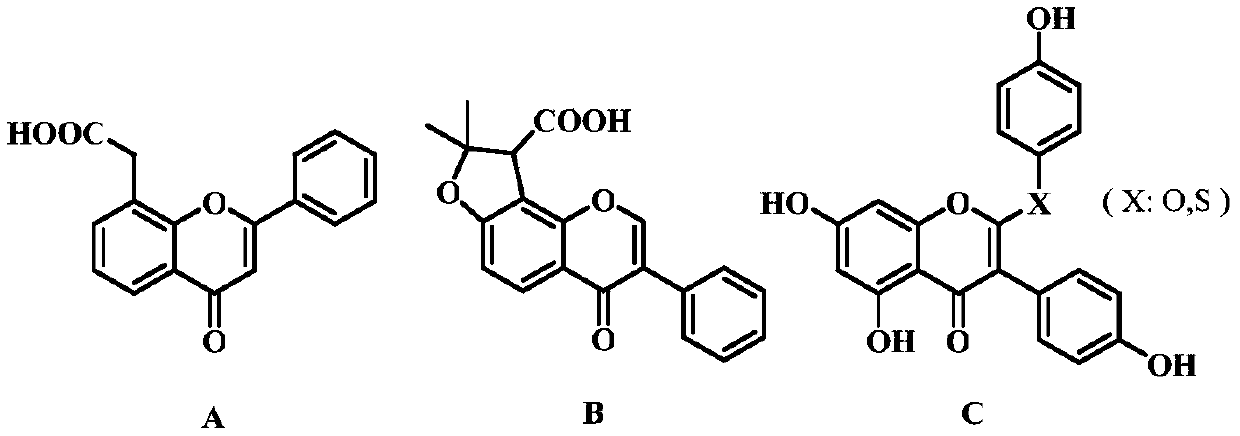
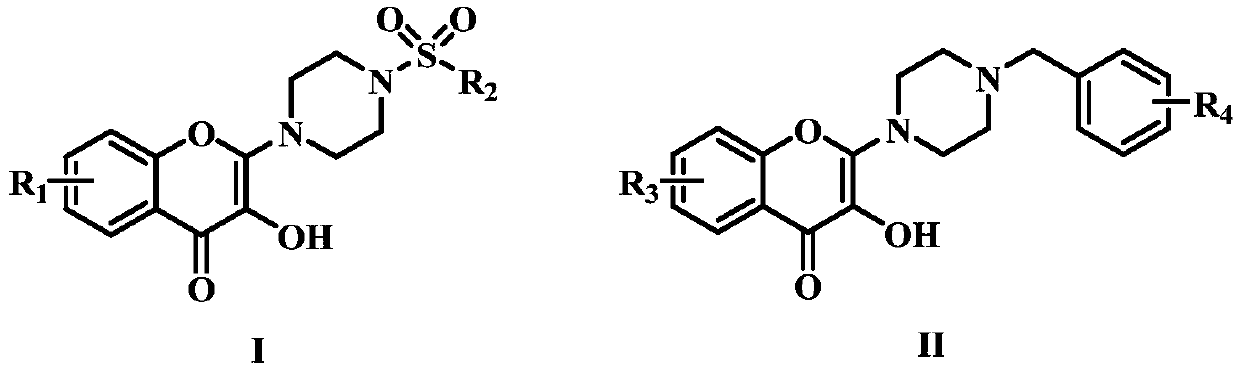
A kind of flavonoid compound and its preparation method and application
A compound and flavonoid technology, applied in the field of anti-tumor compounds, can solve the problems of poor human activity and achieve obvious anti-tumor effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] The preparation method of described flavonoid compound is as follows:
[0040] First, dissolve 2-hydroxyacetophenone and N,N-dimethylformamide dimethyl acetal in dry N,N-dimethylformamide, heat the oil bath to 75°C, and stir for 4h. After extraction with ethyl acetate and separation and purification by silica gel chromatography, intermediate a was obtained. Next, the intermediate a was dissolved in dichloromethane, concentrated hydrochloric acid was added dropwise, and the reaction solution was placed in an oil bath and heated to reflux for 1 h. After extraction with dichloromethane, the organic phases were combined, washed with saturated sodium bicarbonate, dried over anhydrous magnesium sulfate, concentrated under reduced pressure, separated and purified by silica gel chromatography to obtain intermediate b. Again, the intermediate b was dissolved in dichloromethane, hydrogen peroxide and sodium hydroxide were slowly added thereto, and stirred at 0°C. After stirring...
Embodiment 1
[0047] Embodiment 1: the synthesis of intermediate compound a (3-(dimethylamino)-1-(2-hydroxyphenyl)-2-enyl acetone)
[0048]
[0049] Dissolve 2-hydroxyacetophenone (1.00g) and N,N-dimethylformamide dimethyl acetal (1.05g) in 10mL of dry N,N-dimethylformamide, heat the oil bath to 75 ℃, stirred for 4h. After analysis by thin-layer chromatography and monitoring the reaction under ultraviolet light, cool to room temperature after completion, add water and extract three times with ethyl acetate, combine the organic layers, wash with saturated sodium chloride solution, and dry over anhydrous magnesium sulfate. The organic phase was concentrated under reduced pressure, separated and purified by silica gel column chromatography using a system of n-hexane:ethyl acetate=4:1 to obtain an orange-yellow solid with a yield of 80%.
[0050] 1 H NMR (600MHz, DMSO-d 6 ): δ14.52(s,1H),7.94(dd,J=1.8,7.3Hz,1H),7.92(d,J=12.1Hz,1H),7.36(dt,J=1.6,7.7Hz,1H) , 6.78~6.86 (m, 2H), 5.98 (d, J=1...
Embodiment 2
[0051] Embodiment 2: the synthesis of intermediate compound b (chromone)
[0052]
[0053] Compound 1 (1 g) was dissolved in 25 mL of dichloromethane, concentrated hydrochloric acid (4 mL) was added thereto, and the reaction solution was placed in an oil bath and heated to reflux for 1 h. Analyzed by thin-layer chromatography and monitored the reaction under ultraviolet light, cooled to room temperature after the reaction was completed, extracted 3 times with dichloromethane, combined the organic phases, washed with saturated sodium bicarbonate, then dried with anhydrous magnesium sulfate, concentrated under reduced pressure , separated and purified by silica gel chromatography to obtain a red solid with a yield of 90%.
[0054] 1 H NMR (600MHz, CDCl 3 )δ: 8.22(dd, J=1.4,7.9Hz, 1H), 7.86(d, J=6.0Hz, 1H), 7.64~7.71(m, 1H), 7.46(d, J=8.4Hz, 1H), 7.41 (t, J=7.6Hz, 1H), 6.35 (d, J=5.8Hz, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com