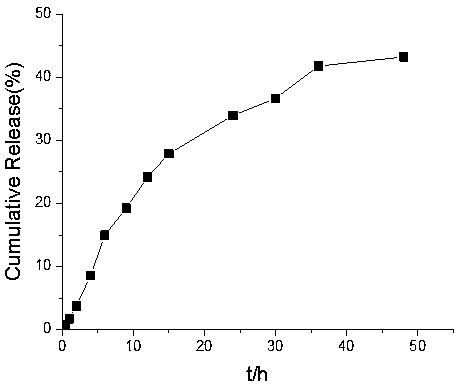
Preparation method and application of reduction-sensitive drug delivery system with high drug loading rate
A technology with high drug loading and delivery system, applied in the field of biomedicine and nanomedicine, can solve the problems of low bioavailability, low pharmacokinetics, obvious side effects, etc., and achieves simple preparation process, stable micelles, and encapsulation. Efficient effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
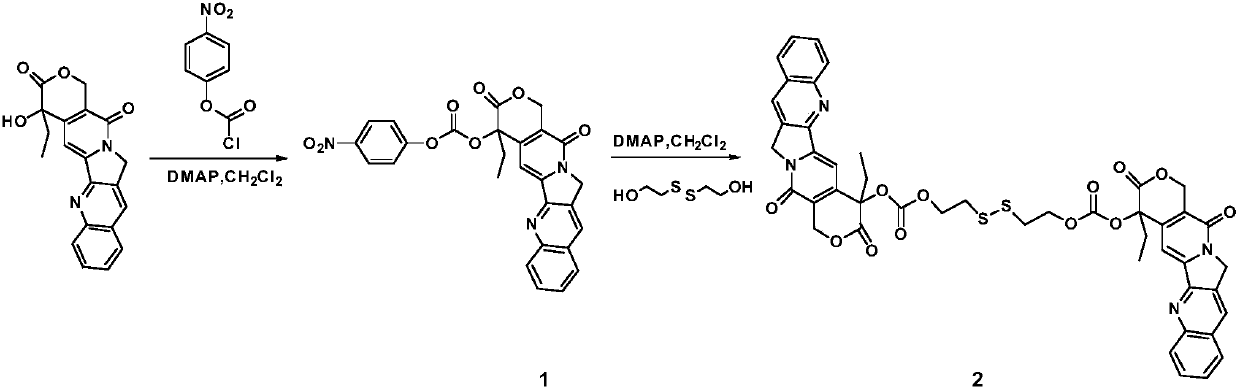
[0024] Add 0.5g camptothecin (1.44mmol), 0.29g p-nitrophenyl chloroformate and 0.39g DMAP in a 100mL Shrek bottle, and dissolve in 25mL anhydrous CH 2 Cl 2 Under the protection of Ar gas, react at room temperature for 2h, wash with 25mL water 3 times, and use anhydrous MgSO for the organic phase 4 Dry and concentrate under reduced pressure to obtain the crude product of Intermediate 1.
Embodiment 2
[0026] The crude product obtained in Example 1 was dissolved in 25 mL of anhydrous CH 2 Cl 2 Add 92.5mg ethyl disulfide in 2.5mL THF and 7.5mL CH 2 Cl 2 The mixed solution was reacted at room temperature for 48h under the protection of Ar gas, filtered, concentrated, and purified by column chromatography (eluent: CH 3 OH / CH 2 Cl 2 ) Approximately 0.23 g of the target product camptothecin dimer was obtained, and the yield was 35.5%.
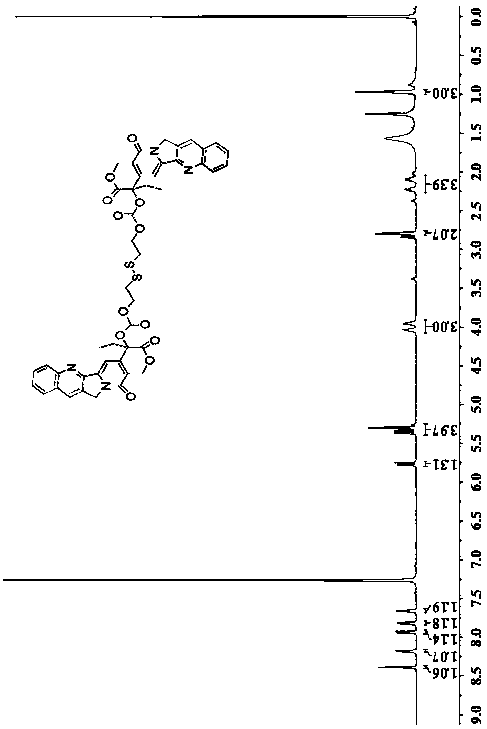
[0027] 1 H NMR(600MHz, CDCl 3 )δ8.39(s,1H), 8.18(d,1H), 7.93(d,1H), 7.82(t,1H), 7.66(t, 1H), 5.76(d,1H), 5.41-5.18(m ,1H),5.46-5.14(m,1H),4.00(d,1H),2.81(d,1H),2.23(d,1H), 2.09(d,1H),0.97(t,1H).ESI- MS for C 46 H 38 N 4 O 12 S 2 [M+H + ] And [M+Na + ]calcd: 902.1928, found: 903.2006 and 925.1786.
Embodiment 3
[0029]
[0030] Combine 0.464g mPEG and 0.204g CL2‰Sn(Oct) 2 Put it into the polymerization tube, dry it in a vacuum drying oven at 38°C, seal the tube when the vacuum is 1Pa, place the reaction in an oil bath at 140°C for polymerization, polymerize for 70h, dissolve the reactants in THF, and transfer to MW= After dialysis in a 14000 dialysis bag, the aqueous phase was lyophilized after dialysis with deionized water for 48 hours to obtain the amphiphilic block copolymer mPEG-b-PCL.
[0031] 1 H NMR(400MHz, CDCl 3 )δ4.05(t,1H), 3.64(s,17H), 3.37(s,1H), 2.39-2.23(m,1H), 1.71-1.57(m,2H),1.45-1.30(m,1H) .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com