Medicine carrier, micelle, anti-tumor and anti-tumor cell metastasis pharmaceutical preparation, and preparation method and use thereof
An anti-tumor cell and anti-tumor technology, applied in the field of pharmaceutical preparations, can solve problems such as metabolism, poor excretion, clinical use restrictions, side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
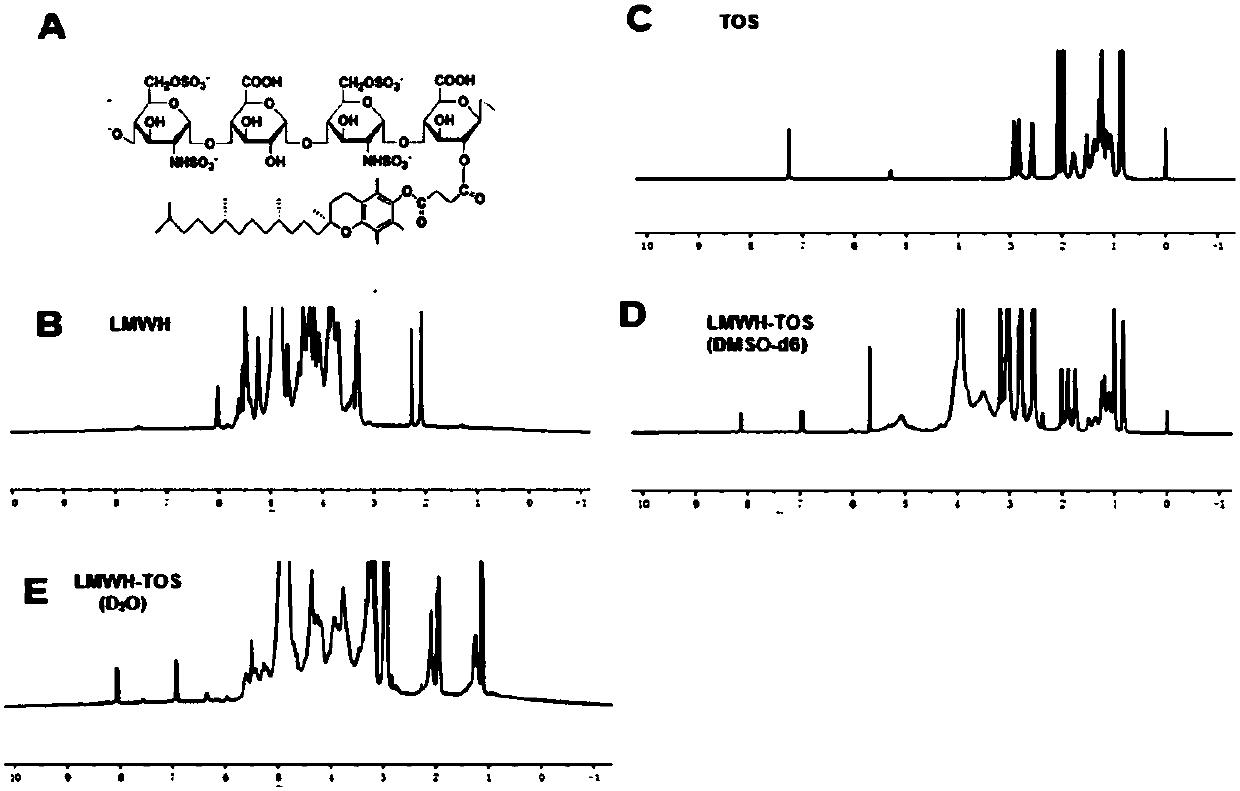
[0229] Synthesis and characterization of embodiment 1 LMWH-TOS copolymer
[0230] The purpose of this example is to illustrate the synthesis method of LMWH-TOS copolymer and the characterization of LMWH-TOS copolymer. In this example, low molecular weight heparin (LMWH) and tocopheryl succinate (TOS) are illustrated by enoxaparin sodium and D-α-TOS respectively, and the prepared copolymers are also used in the following example 2- 6 in.
[0231] (1) Synthesis of LMWH-TOS copolymer
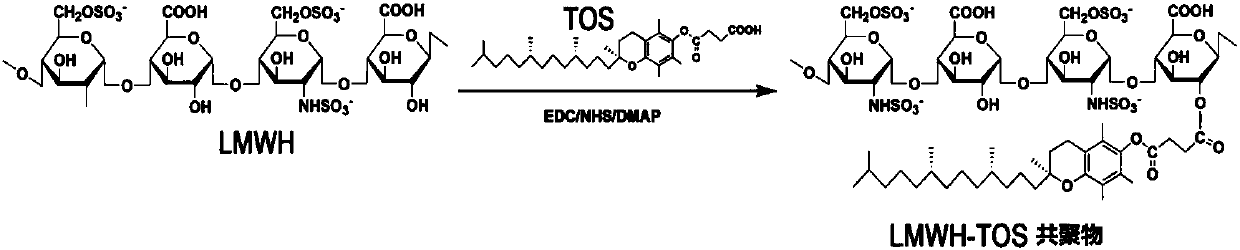
[0232] like figure 1 As shown, enoxaparin sodium and D-alpha-tocopheryl succinate were linked by synthesizing an ester bond that was cleavable by polyesterase in lysosomes at low pH.
[0233] 1. Preparation of enoxaparin sodium and D-α-tocopheryl succinate copolymer (HT)
[0234] (1) Dissolve the D-alpha-tocopherol succinate (403mg) and activator (EDC 253mg, NHS 152mg, DMAP40mg) in 15ml N,N-dimethylformamide (DMF) solvent, avoid Activation for 4 hours under light nitrogen protection; (2) Disso...
Embodiment 2
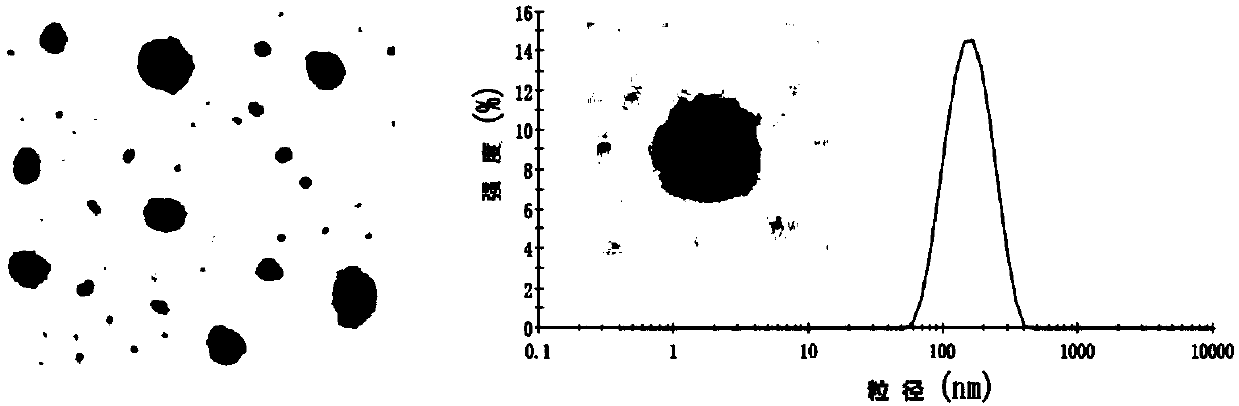
[0244] Example 2 Preparation of HT NPs, DT NPs, FT NPs
[0245] 1. Preparation of HT NPs
[0246] (1) Dissolve the D-alpha-tocopherol succinate (403mg) and activator (EDC 253mg, NHS 152mg, DMAP40mg) in 15ml N,N-dimethylformamide (DMF) solvent, avoid Activated for 4h under the condition of light nitrogen protection;
[0247] (2) Dissolve 200 mg of enoxaparin sodium in 8 ml of formamide solvent, and mix the solutions of the above steps (1) and (2). React at 30-33°C for 72h under the condition of protecting from light and nitrogen. After the reaction, a double-volume acetone precipitation method was used to remove unreacted D-α-tocopheryl succinate and activator to obtain a milky white gel-like precipitate. The above precipitate was dissolved in a small amount of deionized water, put into a dialysis bag with a molecular weight cut-off of 1000, and then lyophilized after dialyzed in deionized water for 48 hours.
[0248] (3) Dissolving the enoxaparin sodium and D-α-tocopheryl ...
Embodiment 3
[0258] The preparation of embodiment 3 PBA-LMWH-TOS micelles
[0259] Taking the targeting molecule 3-aminophenylboronic acid (PBA) as an example, on the basis of the low molecular weight heparin-α-tocopheryl succinate copolymer (LMWH-TOS) prepared as in Example 1, directly through acylation The low molecular weight heparin-α-tocopherol succinate copolymer with targeting components can be prepared by reacting and bonding 3-aminophenylboronic acid (PBA). The specific connection steps are as follows:
[0260] Dissolve 200 mg of the powdered amphiphilic copolymer prepared in Example 1 and an activator (EDC 253 mg / NHS 152 mg) in 8 ml of N,N-dimethylformamide solvent. After activation for 3 hours, add 3-amino 5 mg of phenylboronic acid (PBA) was reacted in the dark under nitrogen protection for 24 hours, the reaction solution was dialyzed in pure water for 72 hours, and freeze-dried to obtain a white powder.
[0261] The white powder prepared above was dissolved in an aqueous mediu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com