Pharmaceutical carrier, micelle, pharmaceutical preparation, and preparation method and use thereof
A drug and carrier technology, applied in the direction of drug combination, pharmaceutical formula, antineoplastic drugs, etc., can solve the problems of metabolism, poor excretion, clinical use restrictions, small drug loading, etc., and achieve simple operation, simple structure, and simple construction Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
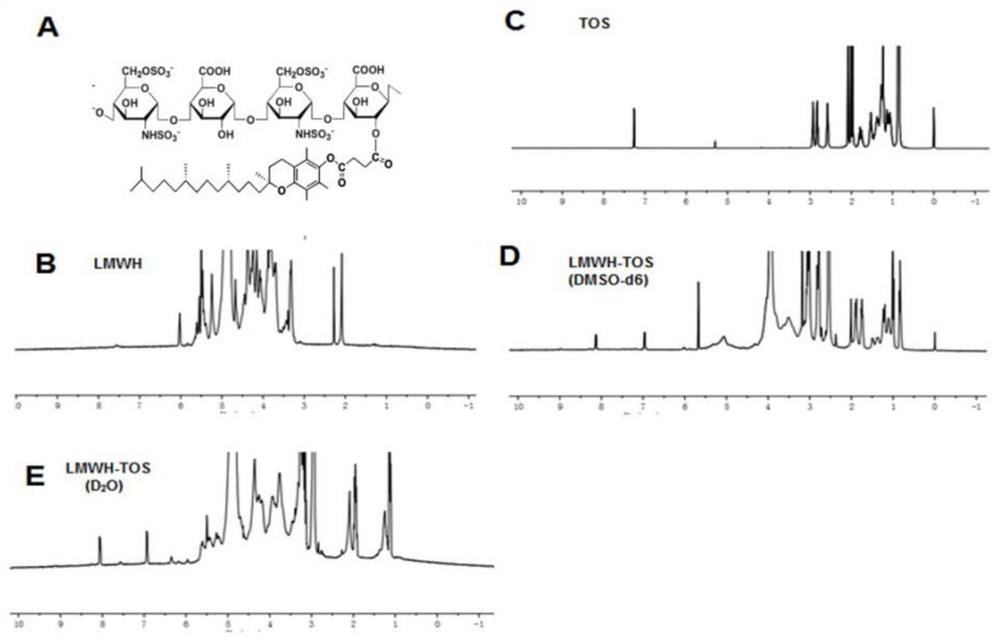
[0229] Synthesis and characterization of embodiment 1 LMWH-TOS copolymer
[0230] The purpose of this example is to illustrate the synthesis method of LMWH-TOS copolymer and the characterization of LMWH-TOS copolymer. In this example, low molecular weight heparin (LMWH) and tocopheryl succinate (TOS) are illustrated by enoxaparin sodium and D-α-TOS respectively, and the prepared copolymers are also used in the following example 2- 6 in.
[0231] (1) Synthesis of LMWH-TOS copolymer
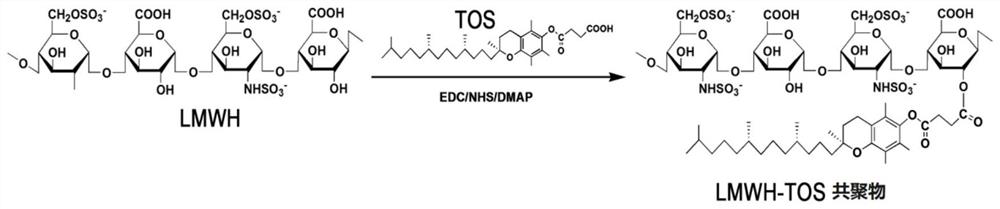
[0232] like figure 1 As shown, enoxaparin sodium and D-alpha-tocopheryl succinate were linked by synthesizing an ester bond that was cleavable by polyesterase in lysosomes at low pH.
[0233] 1. Preparation of enoxaparin sodium and D-α-tocopheryl succinate copolymer (HT)
[0234] (1) Dissolve the D-alpha-tocopherol succinate (403mg) and activator (EDC 253mg, NHS 152mg, DMAP40mg) in 15ml N,N-dimethylformamide (DMF) solvent, avoid Activation for 4 hours under light nitrogen protection; (2) Disso...
Embodiment 2
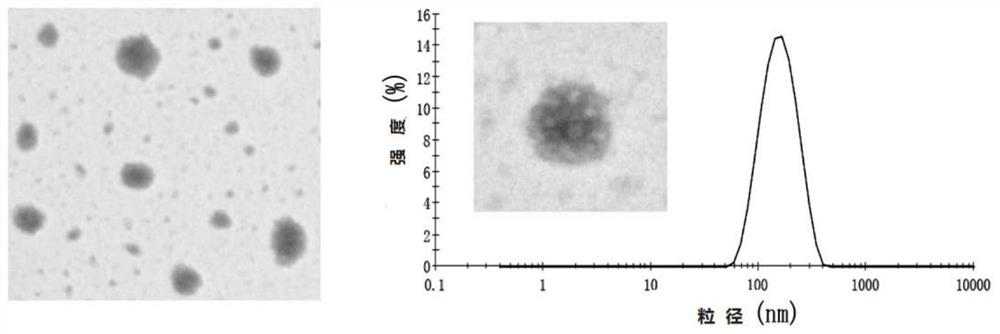
[0244] Example 2 Preparation of HT NPs, DT NPs, FT NPs
[0245] 1. Preparation of HT NPs
[0246] (1) Dissolve the D-alpha-tocopherol succinate (403mg) and activator (EDC 253mg, NHS 152mg, DMAP40mg) in 15ml N,N-dimethylformamide (DMF) solvent, avoid Activated for 4h under the condition of light nitrogen protection;
[0247] (2) Dissolve 200 mg of enoxaparin sodium in 8 ml of formamide solvent, and mix the solutions of the above steps (1) and (2). React for 72 hours at 30-33°C under the protection of light and nitrogen. After the reaction, a double-volume acetone precipitation method was used to remove unreacted D-α-tocopheryl succinate and activator to obtain a milky white gel-like precipitate. The above precipitate was dissolved in a small amount of deionized water, put into a dialysis bag with a molecular weight cut-off of 1000, and then lyophilized after dialyzed in deionized water for 48 hours.
[0248] (3) Dissolving the enoxaparin sodium and D-α-tocopheryl succinate ...
Embodiment 3
[0258] The preparation of embodiment 3 PBA-LMWH-TOS micelles
[0259] Taking the targeting molecule 3-aminophenylboronic acid (PBA) as an example, on the basis of the low molecular weight heparin-α-tocopheryl succinate copolymer (LMWH-TOS) prepared as in Example 1, directly through acylation The low molecular weight heparin-α-tocopherol succinate copolymer with targeting components can be prepared by reacting and bonding 3-aminophenylboronic acid (PBA). The specific connection steps are as follows:
[0260] Dissolve 200 mg of the powdered amphiphilic copolymer prepared in Example 1 and an activator (EDC 253 mg / NHS 152 mg) in 8 ml of N,N-dimethylformamide solvent. After activation for 3 hours, add 3-amino 5 mg of phenylboronic acid (PBA) was reacted in the dark under nitrogen protection for 24 hours, the reaction solution was dialyzed in pure water for 72 hours, and freeze-dried to obtain a white powder.
[0261] The white powder prepared above was dissolved in an aqueous mediu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com