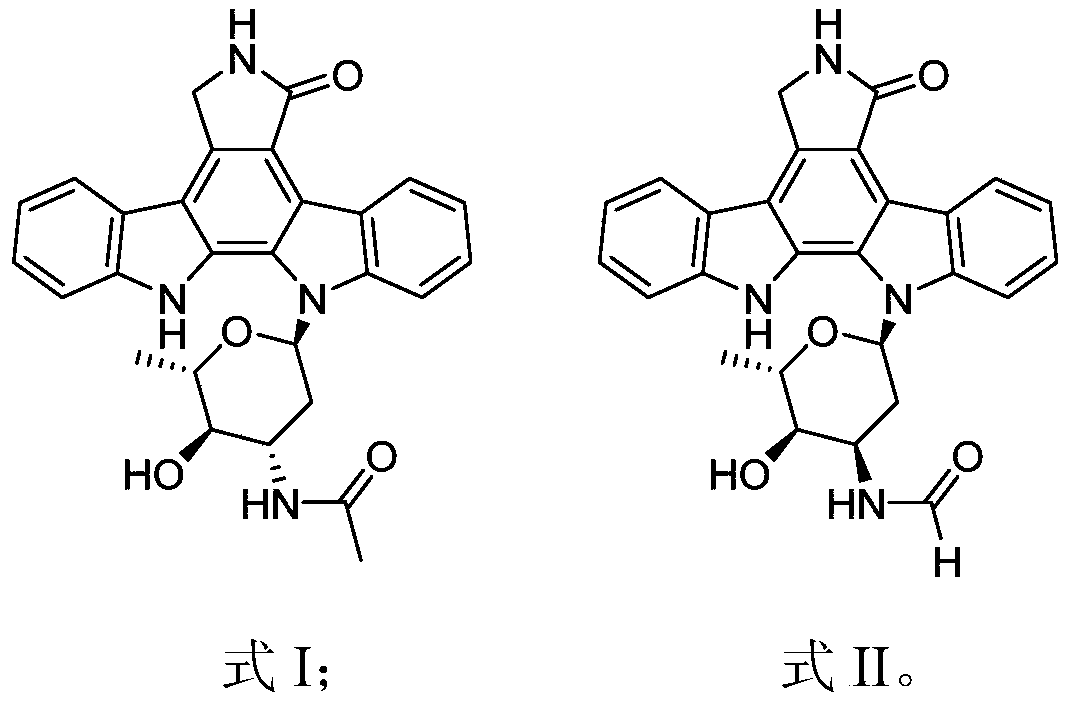
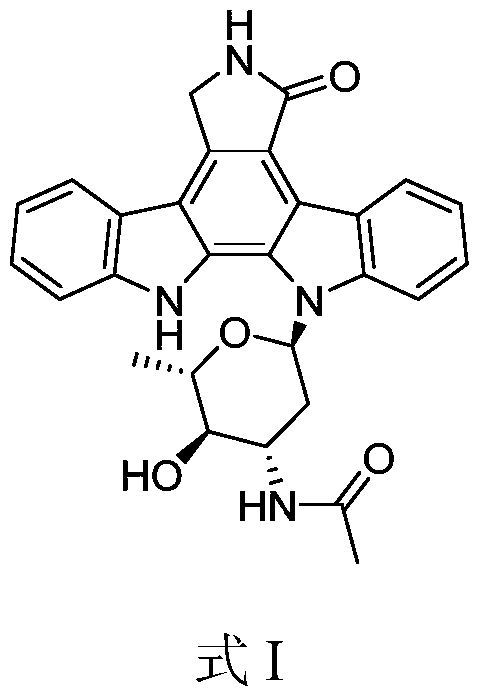
Indolecarbazole compounds and their preparation methods and applications
A technology for indolecarbazoles and compounds, applied in the field of indolecarbazoles and their preparation, capable of solving problems such as poor specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
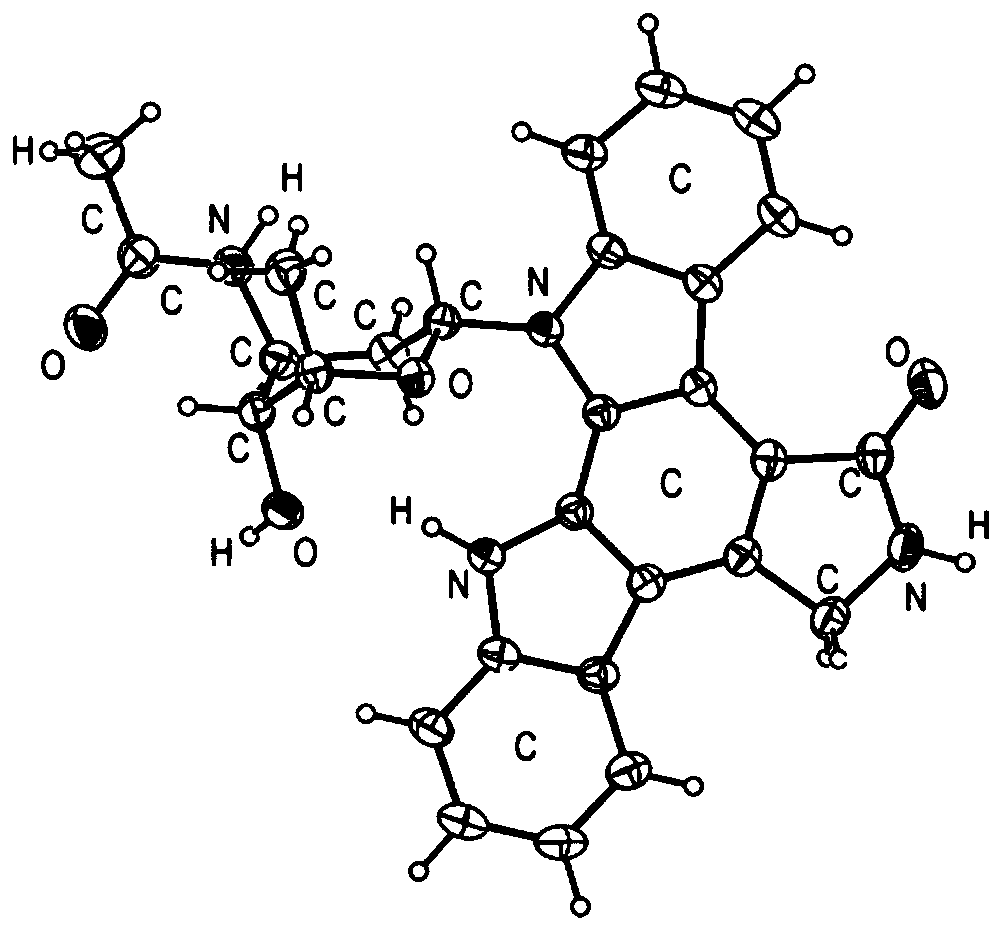
[0039] 1. Fermentation of compounds
[0040] The marine actinomycete adopts the streptomyces Streptomycessp.CICC 11025 sold by China Common Microorganism Culture Collection and Management Center;
[0041] 1) Inoculate marine actinomycetes in 500mL Erlenmeyer flasks, and each bottle contains 250mL Gaoshi No. 1 liquid medium (each liter contains: 10g of soluble starch; 1g of sodium chloride; 0.5g of dipotassium hydrogen phosphate; heptahydrate and Magnesium sulfate 0.5g; Ferrous chloride 0.01g; Sea salt 25g), the culture condition is 28 ℃, 180rpm in the shaker culture for 3 days, obtain the seed liquid that can be used for fermentation culture;
[0042] 2) Inoculate the seed liquid obtained in step 1) into rice medium (rice medium, made of the following components: 40 g of rice mass; 60 mL of seawater, obtained by high-pressure damp heat sterilization after being placed in a 500 ml Erlenmeyer flask), and inoculated The volume is 12 mL, and it is cultured statically at 28° C. fo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| volume ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com