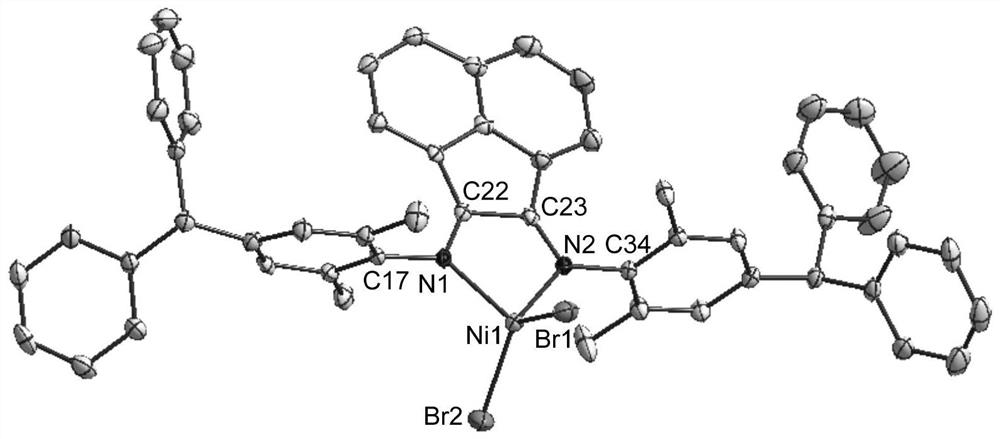
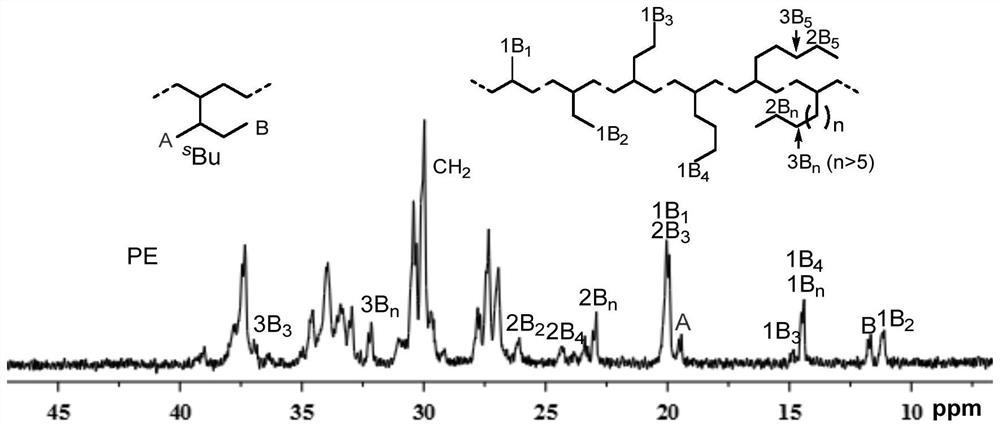
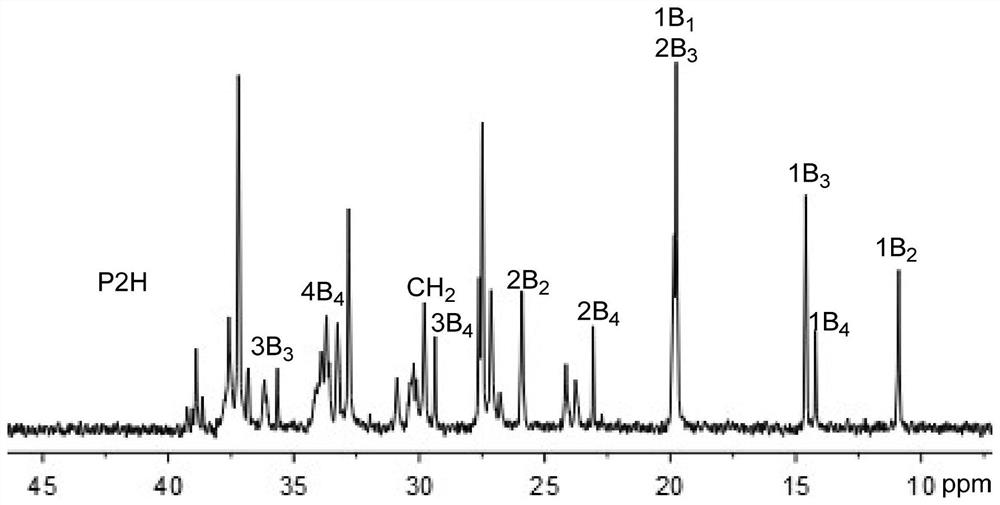
Nickel(ii) complexes containing p-diphenylmethyl-substituted α-diimine for catalyzing the polymerization of ethylene and 2-hexene
A technology of benzhydryl and diimide nickel is applied in the field of olefin polymerization to achieve the effect of high activity and high branching degree
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (1) Synthesis of 4-benzhydryl-2,6-dimethylaniline:
[0035]
[0036] 2,6-Dimethylaniline (1.21g, 10mmol) and diphenylmethanol (1.84g, 10mmol) were placed in a 50ml round-bottomed flask, stirred and heated to 120°C, then anhydrous chloride was added to the mixture Zinc (0.681g, 5mmol), concentrated hydrochloric acid (37%, H 2 O, 0.365g, 10mmol), heated to 160°C, reacted for 1 hour (exothermic and vigorous bubbling), after cooling to room temperature, the solid was dissolved in CH 2 Cl 2 (70 mL), washed with saturated NaOH aqueous solution, washed with anhydrous MgSO 4 Dry, concentrate under reduced pressure, add a large amount of ethanol to precipitate a white solid, filter and dry to obtain 2.33 g of 4-benzhydryl-2,6-dimethylaniline, and the yield is 81%.
[0037] 1 H NMR (500MHz, CDCl 3 ,ppm):δ7.33–7.29(m,4H,C9),7.25–7.22 (m,2H,C10),7.17(d,J=7.0Hz,4H,C8),6.74(s,2H,C3) ,5.46(s, H,C6),3.50(s,2H,-NH 2 ), 2.16(s,6H,C5). 13 C NMR (125MHz, CDCl 3 ,ppm): δ144.75(C6)...
Embodiment 2
[0056] (1) Synthesis of 4-benzhydryl-2,6-diisopropylaniline:
[0057]
[0058] 2,6-diisopropylaniline (1.77g, 10mmol) and diphenylmethanol (1.84g, 10mmol) were placed in a 50ml round bottom flask, and after heating up to 120°C, anhydrous zinc chloride was added to the mixture (0.681g, 5mmol), concentrated hydrochloric acid (37%, H 2 O, 0.365g, 10mmol), the temperature was raised to 160°C, and the reaction was carried out for 1 hour (exothermic and strong bubbling). After cooling to room temperature, the solid was dissolved in CH 2 Cl 2 (60 mL), washed with saturated NaOH aqueous solution, washed with anhydrous MgSO 4 Dry, concentrate under reduced pressure, add a large amount of ethanol to precipitate a white solid, filter and dry to obtain 2.85 g of 4-benzhydryl-2,6-dimethylaniline, and the yield is 83%.
[0059] 1 H NMR (500MHz, CDCl 3 ,ppm):δ7.32–7.29(m,4H,C8),7.24–7.21(m,2H,C9),7.16(d,J=7.5Hz,4H,C7),6.84(s,2H,C3) ,5.50(s,H,C5),3.02(s,2H,-NH 2 ),2.21(s,4H,C10),1....
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com