PD-1 gene silenced CD133-targeting CAR T cell and application thereof
A gene silencing and PD-1 technology, applied in the field of CART cells targeting CD133, can solve the problems of systemic toxicity, difficulty in antibody development, long time and investment, etc., to achieve precise regulation of the immune microenvironment and prevention of tumor metastasis and recurrence, avoiding the effect of systemic toxicity problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Isolation and preparation of PBMC:
[0059] 1. Collect peripheral blood from healthy people (or patients) with an anticoagulant tube. After diluting the anticoagulated blood (1:1) with PBS, slowly add it into a 50ml centrifuge tube filled with an equal volume of lymphocyte separation medium (Ficoll), and centrifuge slowly at a centrifugal force of 450g for 25min;
[0060] 2. After centrifugation, carefully absorb the buffy coat layer above the lymphocyte separation solution, transfer it to a new 50ml centrifuge tube, add PBS, and centrifuge slowly at a centrifugal force of 300g for 10 minutes, discard the supernatant, and keep the centrifuge tube The cell pellet at the bottom; add PBS again, centrifuge slowly with a centrifugal force of 160g for 15min, discard the supernatant; finally add PBS, centrifuge slowly with a centrifugal force of 300g for 10min, discard the supernatant, and obtain PBMC.
Embodiment 2
[0062] Preparation of CAR T cells:
[0063] 1. After slightly culturing PBMC with T cell culture medium (RPMI 1640 medium containing 10% FBS) for 1 to 2 hours, conduct electroporation, and transfect the following two groups respectively:
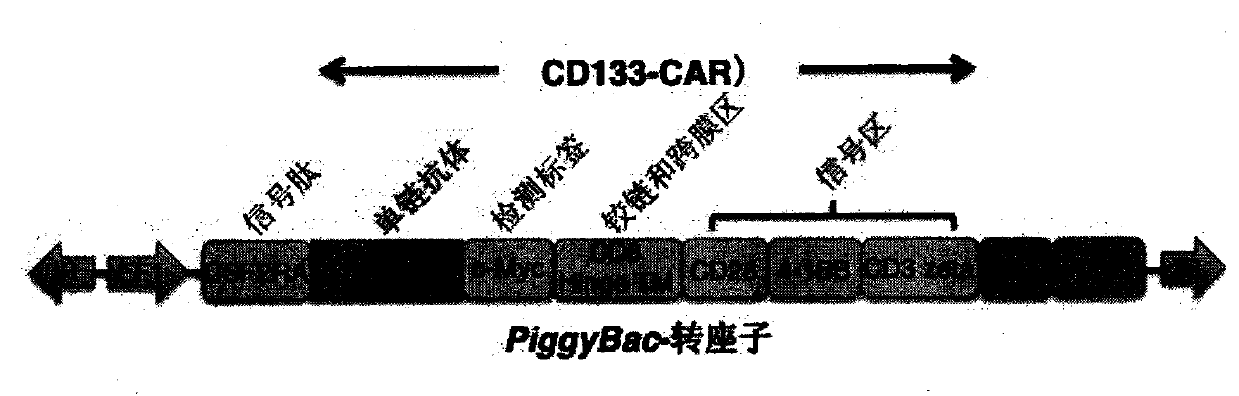
[0064] (1) pGL3-U6-hPD1-sgRNA (1+2) (structure diagram as shown in figure 2 shown), pST1374-NLS-flag-Cas9-ZF, AC133-CAR piggyBac transposon (structure shown in figure 1 Shown) and Super piggyBactransposase 5 μg each of the four plasmids were mixed with the electroporation buffer in the human T cell nucleofection kit (Lonza, VPA-1002) to obtain about 110 μl electroporation mixture containing the four plasmids;
[0065] (2) Use the electroporation mixture containing the original plasmids pGL3-U6-sgRNA, pST1374-NLS-flag-Cas9-ZF, AC133-CARpiggyBac transposon and Super piggyBactransposase as a control; leave some cells without electroporation;
[0066] The preparation methods of the plasmids pGL3-U6-hPD1-sgRNA (1+2), pST1374-NLS-flag-Cas9-ZF and...
Embodiment 3
[0073] Detection of CAR expression by flow cytometry
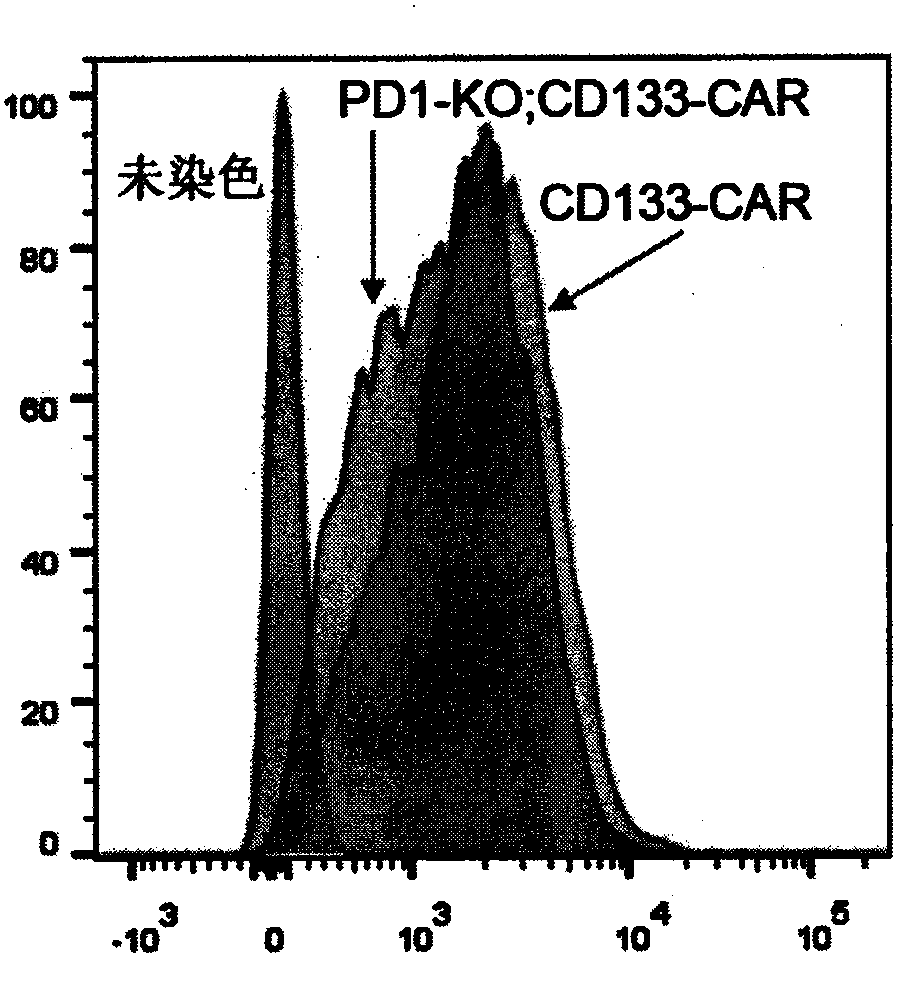
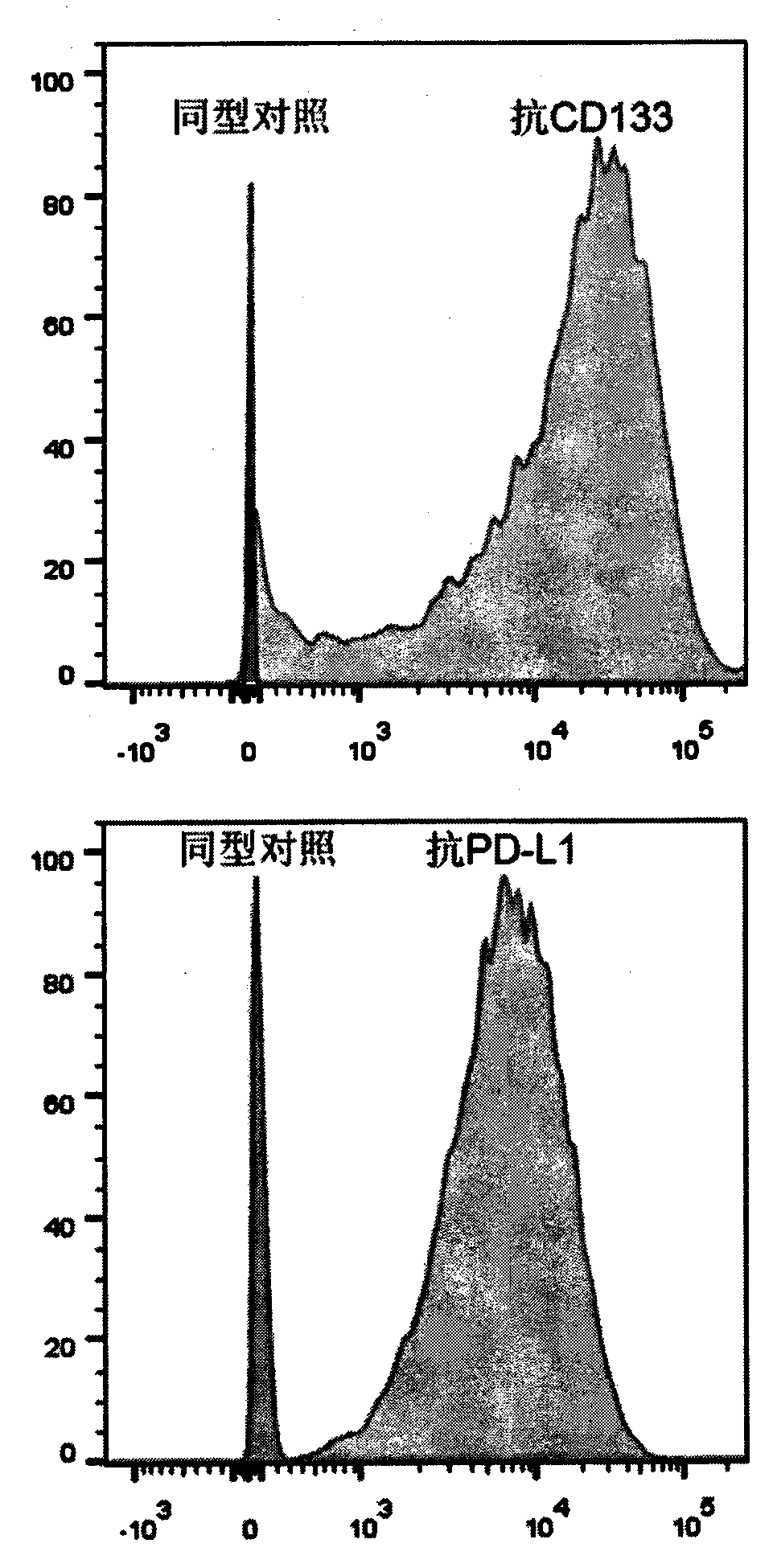
[0074] will be 10 5 The expanded CAR-T cells were resuspended in 100 microliters of FACS buffer (PBS containing 2mM EDTA and 0.5% BSA), and Myc-Tag (9B11) Mouse mAb (1:500 dilution, Cell Signaling, 2276S), incubated at 4°C for 15 minutes; washed once, resuspended in 100 microliters of FACS buffer, added PE-labeled goat anti-mouse secondary antibody (1:50 dilution, Jackson, 115-116-072), Incubate at 4°C for 10 minutes; CytoFLEX (Beckman Coulter) flow cytometer is used to obtain stained cells, and FlowJo is used to analyze the results. like image 3 As shown, flow cytometry analysis showed that cells such as traditional CD133-CAR T and PD-1 knockout CD133-CAR T (PD1-KO; CD133-CAR) highly expressed CD133-CAR molecules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com