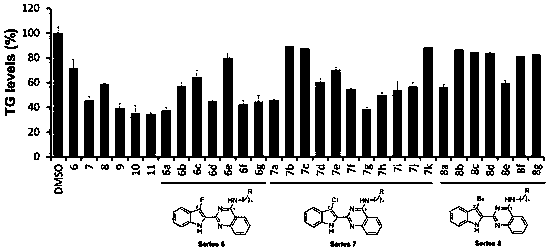
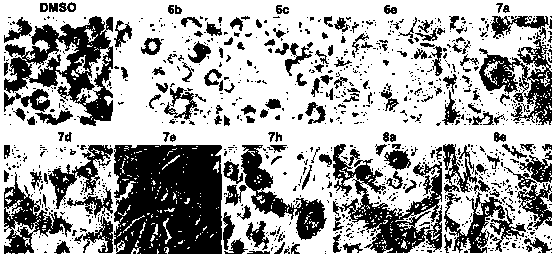
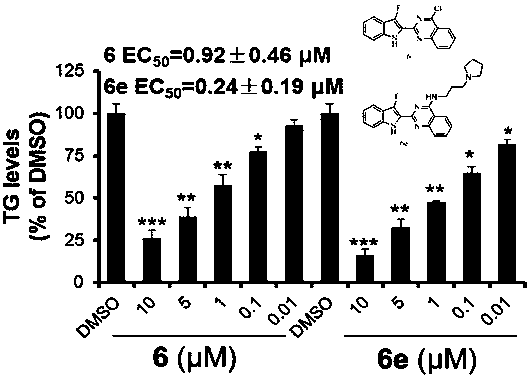
Anti-obesity compound and preparing method and application thereof
A compound and obesity technology, applied in organic chemistry, drug combination, metabolic diseases, etc., can solve the problems of high toxicity and poor safety, and achieve the effect of low toxicity, short reaction time and good lipid-lowering activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1: AOCN1
[0060] 5-Methoxyindole-2-carboxylic acid (0.96g, 5mmol) was dissolved in a round bottom flask containing 10mL of dehydrated dichloromethane, and oxalyl chloride (1.27mL, 15mmol) and 2 Drop N,N-dimethylformamide as a catalyst. After 2 hours of reaction, it turns from beige turbidity to dark yellow clarity. After spinning the solvent, it is dissolved in tert-butanol, and 0.68g of anthranilamide is added, and it immediately turns yellow turbidity. , followed by adding potassium tert-butoxide (1.12g, 10mmol), the solution gradually turned orange-red turbid, the temperature was slowly raised from 25°C to 100°C for about 8 hours, and the reaction was stopped. After the reaction solution was cooled to room temperature, it was filtered The filtrate was obtained, and the filtrate was poured into ice water, and a large amount of solids were precipitated by ultrasonication. The pH was adjusted to about 8 with dilute hydrochloric acid, and 1.02 g of a yellow soli...
Embodiment 2
[0066] Example 2: IQN4
[0067] Add 14.4g of anthranilamide into tert-butanol dissolved with 2-indole chloride at room temperature, at this time, the reaction solution turns from brown clear to light yellow turbid; then add 2.4 equivalents of potassium tert-butoxide , the temperature was slowly raised from room temperature to 80°C, and reacted for 9 hours, and the reaction solution turned reddish brown and turbid. Stop the reaction, cool and let it stand, pour into a large amount of ice water, adjust the pH to about 8 with dilute hydrochloric acid, a large amount of solid precipitates, filter and dry to obtain 21.56g of yellow-white solid (intermediate IQ4).
[0068] POCl 3 and DMF with POCl 3 : The equivalent of DMF = 2.25: 1 was used to generate Vilsmeier reagent under ice bath conditions, then slowly add 1g IQ4 to Vilsmeier reagent, and react at room temperature for 1h, the solution gradually turned reddish brown. Stop the reaction, slowly drop water into the reaction so...
Embodiment 3
[0073] Example 3: DS-B6
[0074] Stir 1.6 g (10 mmol) of indole-2 carboxylic acid in 8 mL of chloroform, slowly add SOCl in an ice-water bath 2 (2.2mL, 30mmol), reacted at 70°C for 1h, removed the solvent under reduced pressure, prepared 8mL of chloroform solution, and slowly added dropwise to the solution containing (1.2mL, 15mmol) pyridine and 3-amino-2-thiophenecarboxylic acid A solution of methyl ester (1.4g, 9mmol) in 8mL of chloroform was reacted at room temperature for 8h. After the reaction, it was allowed to stand, filtered, and air-dried to obtain 2.55 g of a yellow-white solid (intermediate DS1a).
[0075] Stir DS1a (0.3g, 2mmol) in 10mL of ethanol, add 2mo / L NaOH solution, and heat up to 80°C for 1h. After the reaction, the reaction solution was cooled to room temperature, poured into ice water, adjusted to acidic pH with 10% HCl, extracted with ethyl acetate, spin-dried the solvent, and the crude product was washed with a small amount of methanol to obtain 283 m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com