Preparation method of nitrogen doped platinum-nickel bimetallic catalyst for ethanol oxidation with cubic structure
A technology of ethanol oxidation and cubic structure, applied in structural parts, electrical components, battery electrodes, etc., can solve the problems of low stability of platinum catalyst, easy poisoning, high price, etc. The effect of low onset potential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Specific steps are as follows:
[0014] (1) At room temperature, mix 2g glucose, 0.8g urea, 0.3g nickel nitrate and 0.5mL chloroplatinic acid solution with a concentration of 7.91mg / mL in a beaker, add 50mL deionized water, and then put it in an oven to dry 12h;
[0015] (2) At room temperature, move the dried sample to a graphite tank, then put it into a vacuum tube furnace for calcination, the heating rate is 5°C / min, the calcination temperature is 900°C, and the holding time is 3h;
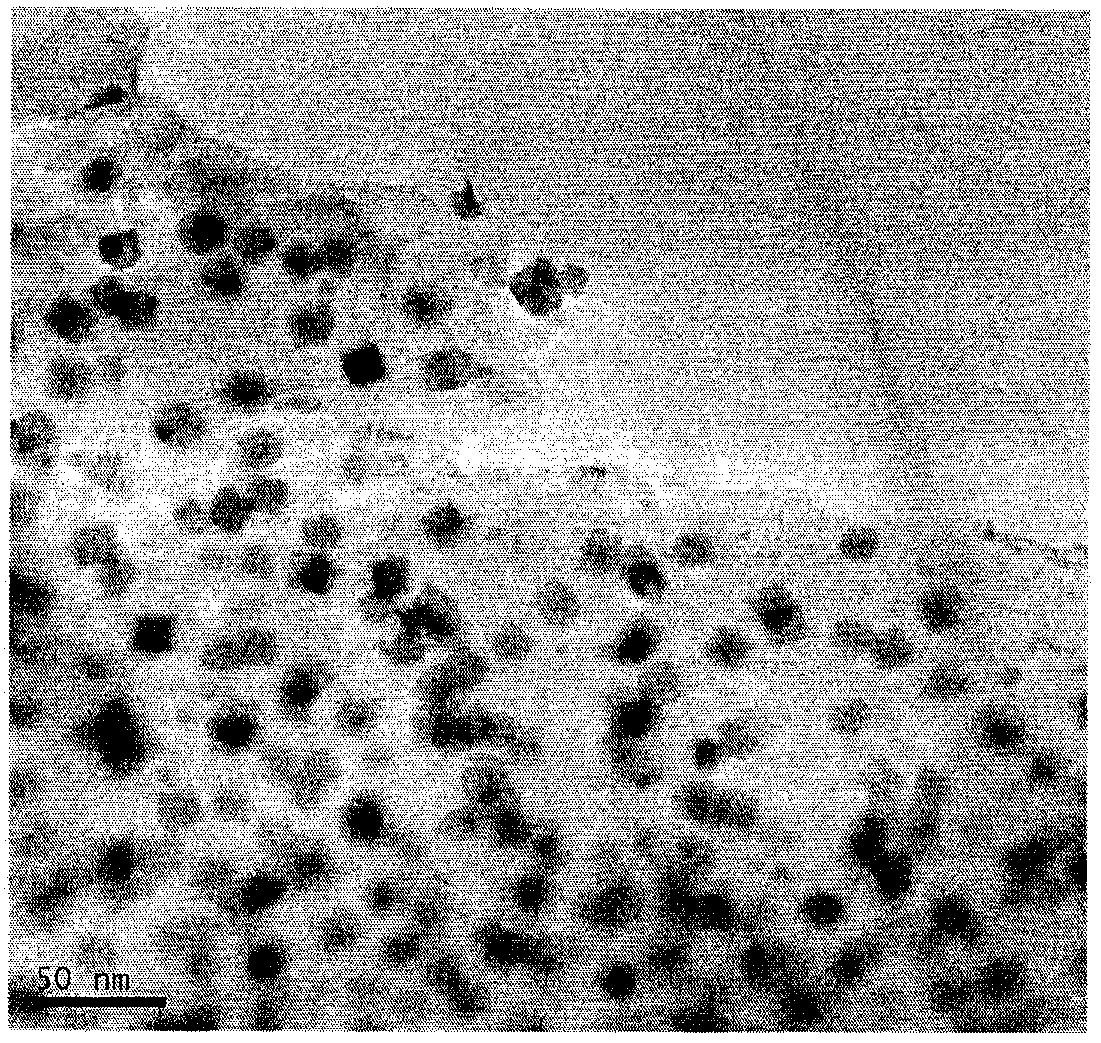
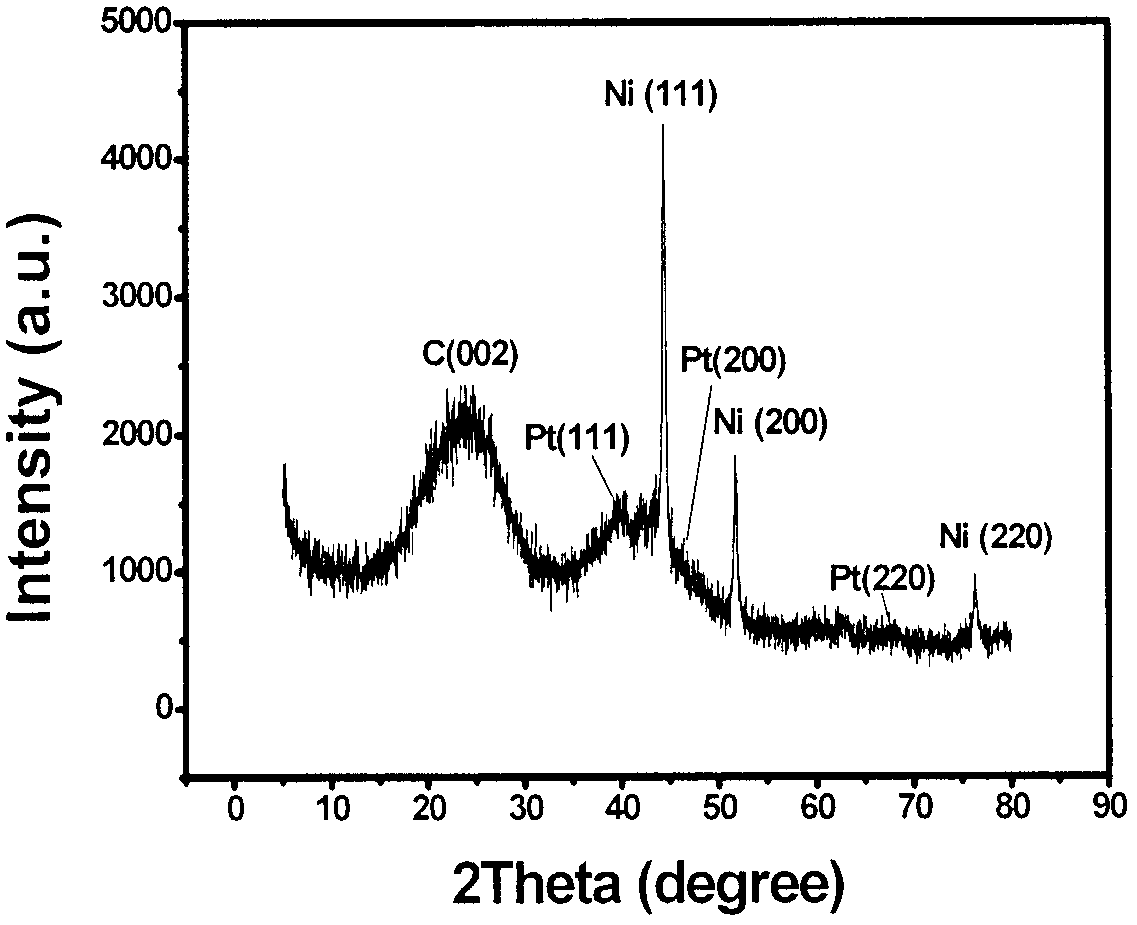
[0016] (3) Grinding the calcined sample into particle-free powder to obtain a nitrogen-doped platinum-nickel bimetallic catalyst. figure 1 It is the HRTEM image of the prepared nitrogen-doped platinum-nickel bimetallic catalyst, which shows that the catalyst has a cubic structure with uniform particle size and uniform distribution. figure 2 The XRD pattern of the prepared nitrogen-doped platinum-nickel bimetallic catalyst shows that both platinum and nickel are successfully loaded int...
Embodiment 2
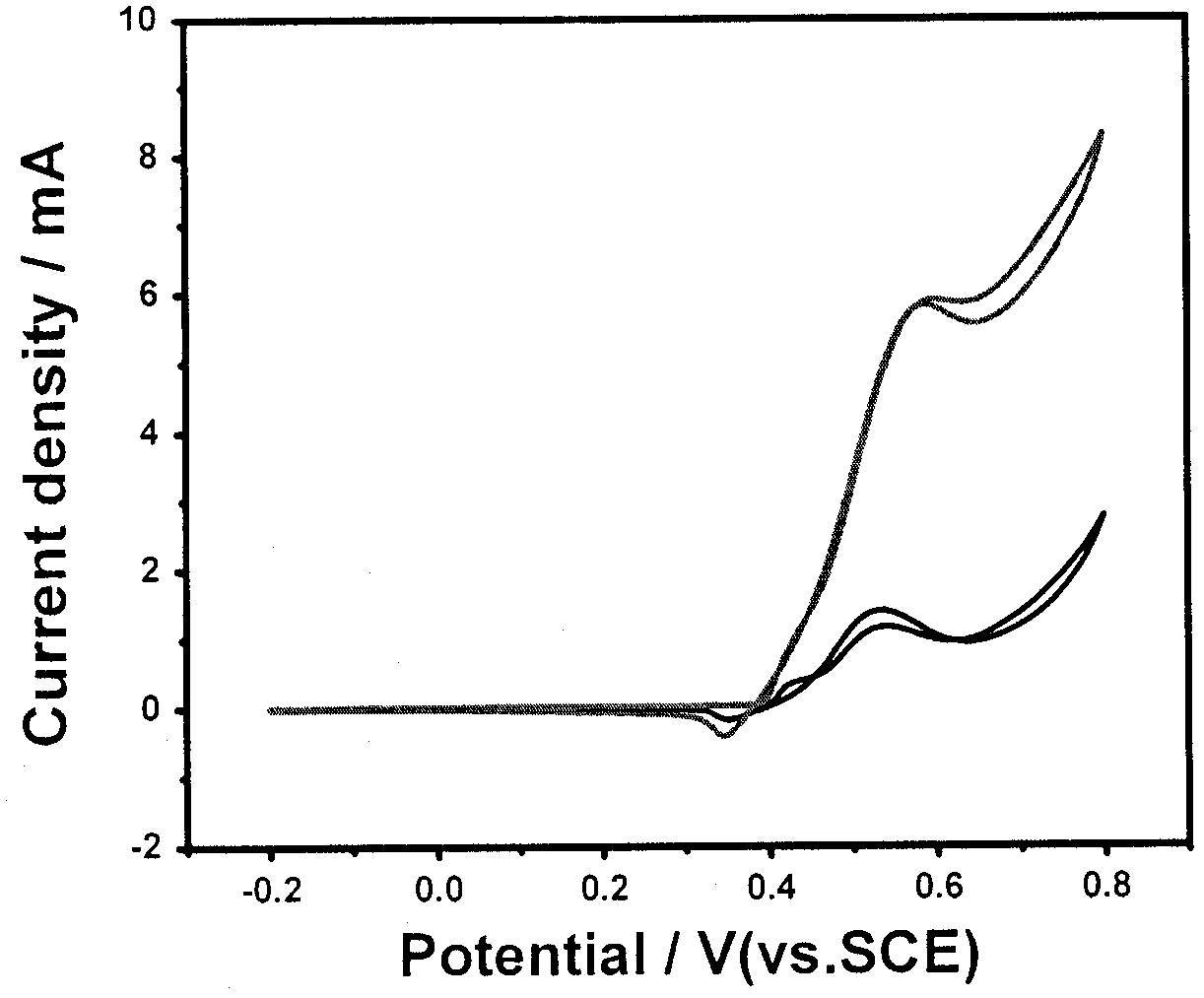
[0018] The same as in Example 1, except that the amount of chloroplatinic acid solution was changed to 0.3 mL. The structure and morphology of the catalyst thus prepared are irregular, the dispersion is not uniform, and compared with Example 1, the current density is lower.
Embodiment 3
[0020] Same as Example 1, except that the amount of chloroplatinic acid solution was changed to 0.1 mL. Compared with Example 1, the catalyst thus prepared has higher onset potential and larger particle size.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com