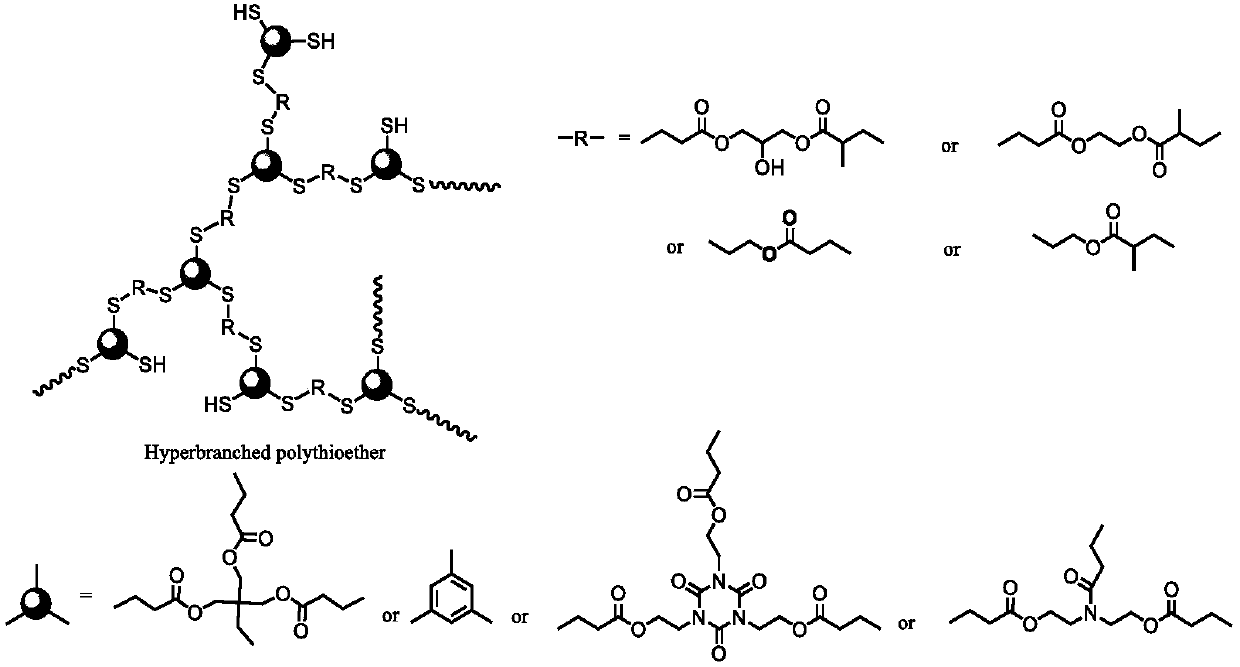
Hyperbranched polythioether preparation method
A technology of polysulfide and thiol, which is applied in the field of preparation of hyperbranched polysulfide, can solve the problems of few types of monomers, prone to cross-linking, poor selectivity, etc. Highly controllable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
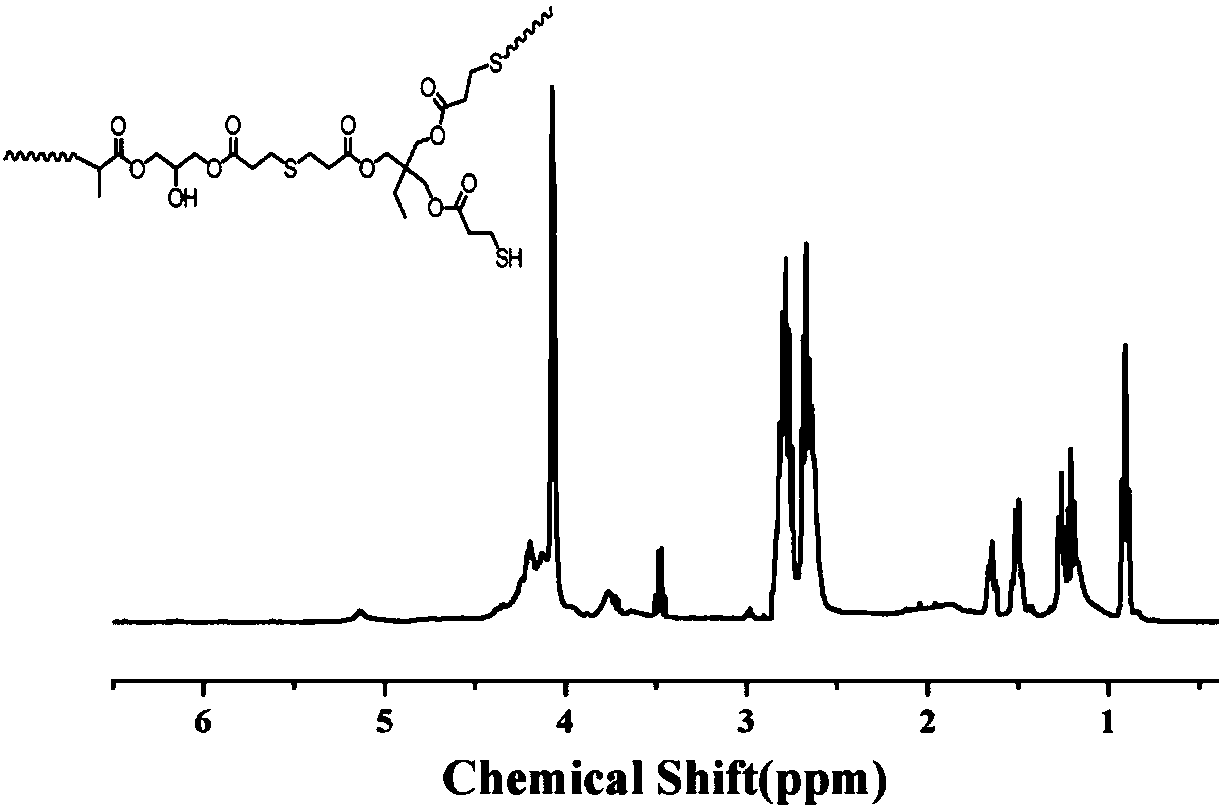
[0023] 9.965g (25mmol) 2-ethylglycerol trimercaptopropionate, 75mL tetrahydrofuran and 5.355g (25mmol) 3-(acryloyloxy)-methacrylic acid-2-hydroxypropyl ester and 0.1265g (1.25 mmol) triethylamine was added to the reactor at one time, and N at 40°C 2 Protect the reaction for 48 hours; after the reaction, the reaction solution was concentrated by rotary evaporation, redissolved in chloroform and precipitated in anhydrous ether, and the dissolution-precipitation operation was repeated 3 times, and the precipitate was vacuum-dried to obtain a colorless viscous liquid product hyperbranched polysulfide (12.68 g, 82.8% yield).
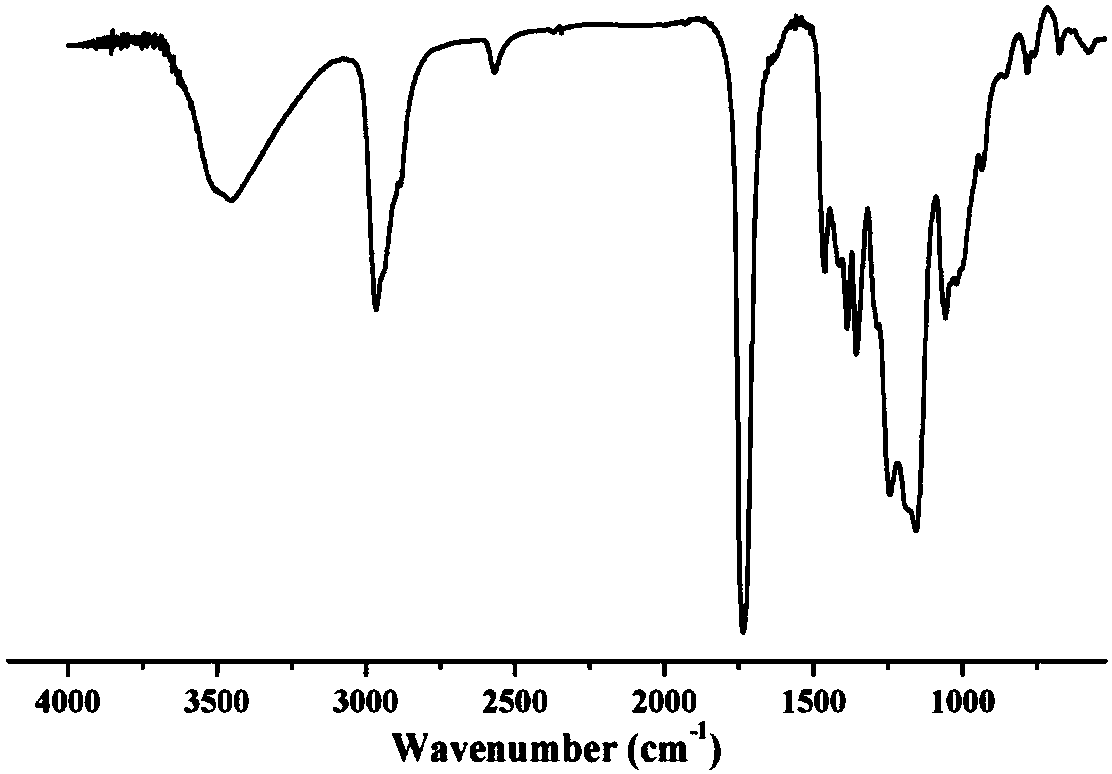
[0024] figure 2 It is the infrared absorption spectrogram of the hyperbranched polysulfide prepared in Example 1, wherein the 3505cm-1 place is the stretching vibration absorption peak of OH, the 2540cm-1 place is the characteristic absorption peak of thiol, and the double bond characteristic at 1600cm-1 place The peaks disappeared completely, proving the ...
Embodiment example 2
[0028] 9.965g (25mmol) 2-ethylglycerol trimercapto propionate, 75mL dioxane and 5.355g (25mmol) 3-(acryloyloxy)-methacrylic acid-2-hydroxypropyl ester and 0.1265 g (1.25mmol) triethylamine was added to the reactor at one time, and N at 40°C 2 Protect the reaction for 48 hours; after the reaction, the reaction solution was concentrated by rotary evaporation, redissolved in chloroform and precipitated in anhydrous ether, and the dissolution-precipitation operation was repeated 3 times, and the precipitate was vacuum-dried to obtain a colorless viscous liquid product hyperbranched polysulfide (12.44 g, 81.2% yield).
Embodiment example 3
[0030] 10.961g (27.5mmol) 2-ethylglycerol trimercaptopropionate, 75mL tetrahydrofuran and 5.355g (25mmol) 3-(acryloyloxy)-methacrylic acid-2-hydroxypropyl ester and 0.1265g ( 1.25mmol) triethylamine is added in the reactor at one time, and under 40 ℃, N 2 Protect the reaction for 24 hours; after the reaction, the reaction solution was concentrated by rotary evaporation, redissolved in chloroform and precipitated in anhydrous ether, and the dissolution-precipitation operation was repeated 3 times, and the precipitate was vacuum-dried to obtain a colorless viscous liquid product hyperbranched polysulfide (11.13 g, 68.2% yield).
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
| Branching factor | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com