DOPO (9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide) derivative fire retardant and preparation method and application thereof
A derivative and flame retardant technology, applied in the field of DOPO derivative flame retardant and its preparation, can solve the problems of poor structural strength and compactness of carbon layer, decreased mechanical and mechanical properties, weak oxygen and heat insulation ability, etc. The effects of mechanical and mechanical properties, enhanced oxygen and thermal insulation capabilities, and enhanced compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Preparation of DOPO derivative 1# of structural formula (1), there is 1 DOPO residue in this DOPO derivative:
[0069]
[0070] The synthetic route of DOPO derivative 1# is as follows:
[0071]
[0072] The synthesis method of DOPO derivative 1# is as follows:
[0073] Step 1 prepares DOPO-containing phenol derivatives 1
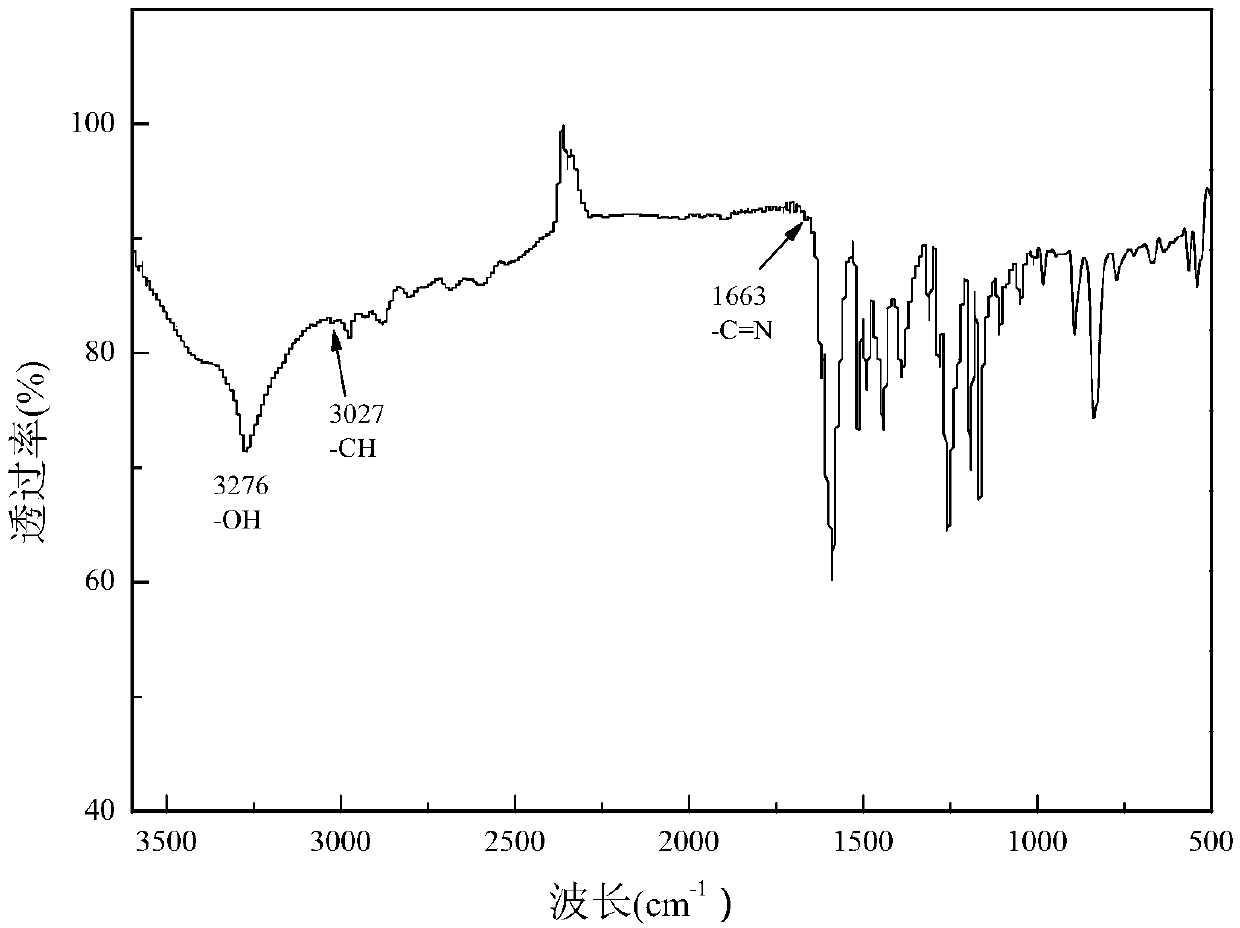
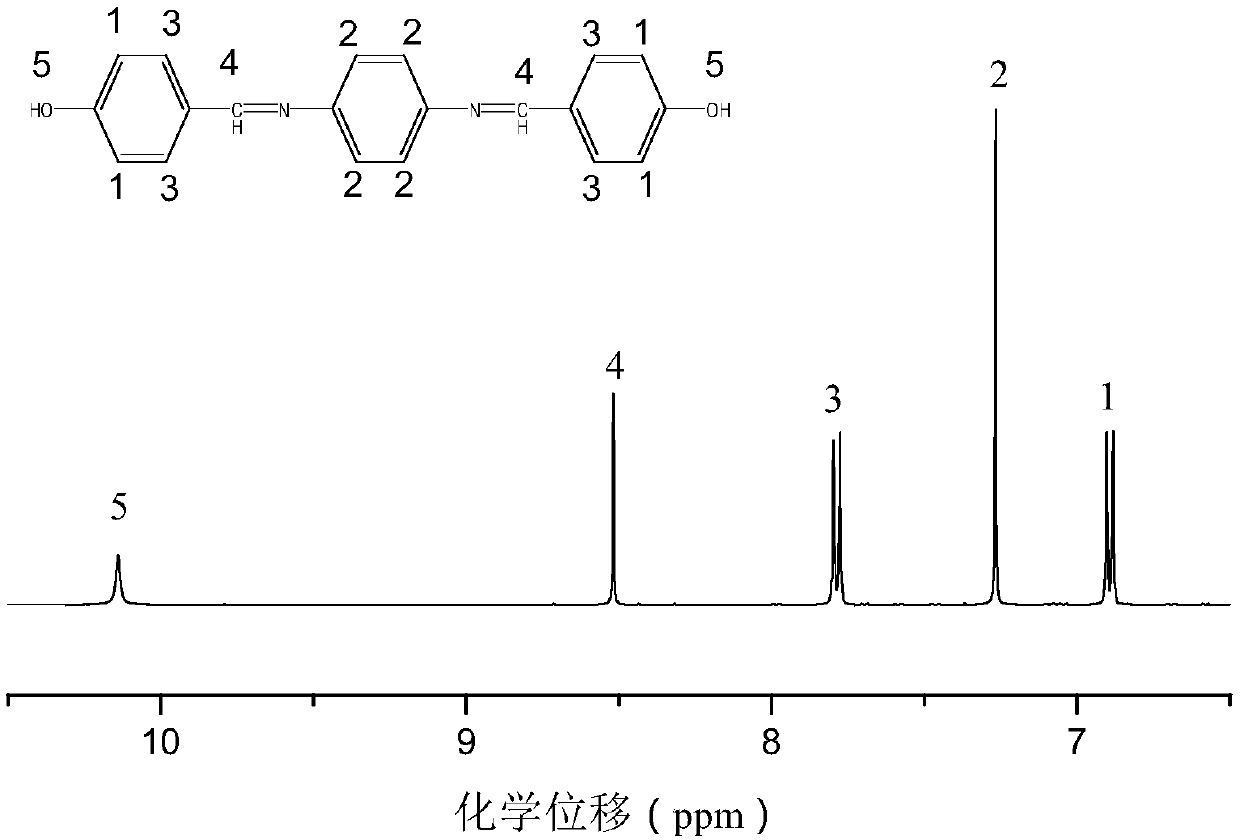
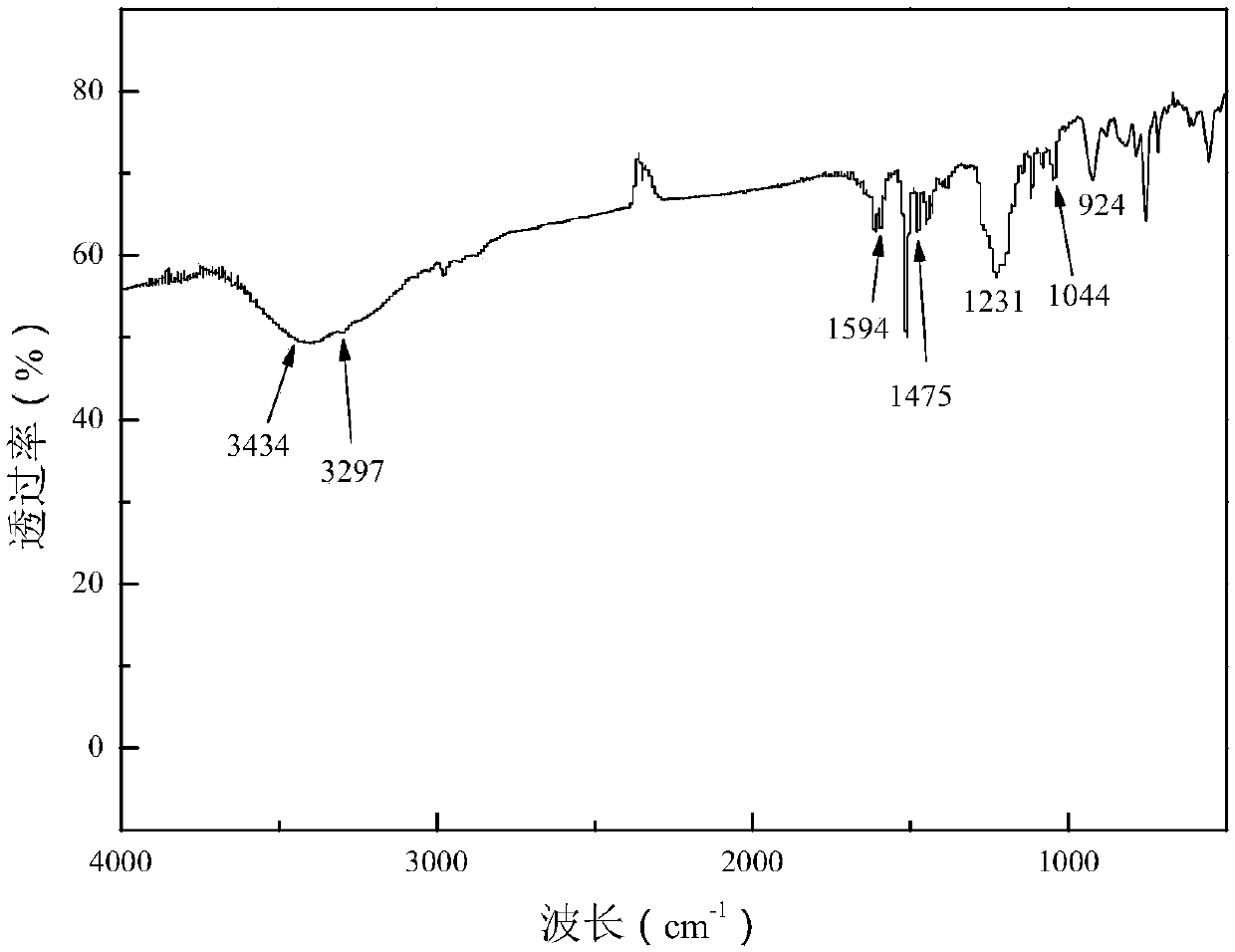
[0074] Add 12.2 g of p-hydroxybenzaldehyde and 10.9 g of p-aminophenol into a three-neck round bottom flask equipped with a magnetic stirrer, and then add 100 ml of methanol as a solvent. in N 2 The mixture was reacted at 50° C. for 6 hours in the atmosphere. The resulting aromatic group-containing Schiff base 1 (10.65g) and 21.6g of DOPO were dissolved in 100ml of tetrahydrofuran (THF), then in N 2 Under protective conditions, it was heated to 60°C and stirred for 12 hours to obtain a precipitate. The resulting precipitate was filtered and washed several times with THF at room temperature, then dried in a vacuum oven. The white product ob...
Embodiment 2
[0080] Prepare the DOPO derivative 2# of structural formula (2), there are 2 DOPO residues in this DOPO derivative:
[0081]
[0082] The synthetic route of DOPO derivative 2# is as follows:
[0083]
[0084]
[0085]
[0086] The synthetic method of DOPO derivative 2# is as follows:
[0087] Step 1 Preparation of DOPO-containing phenol derivatives 2
[0088] Add p-hydroxybenzaldehyde and p-phenylenediamine in a molar ratio of 2:1 (24.4g and 10.8g respectively) into a three-neck round bottom flask equipped with a magnetic stirrer, and then add 300ml of methanol as a solvent. in N 2 The mixture was reacted at 60° C. for 6 hours in the atmosphere, and the resulting precipitate was filtered and washed. Dry in an oven to obtain the Schiff base 2 containing aromatic groups. A mixture of the obtained product (31.6 g), 43.2 g of DOPO and 250 ml of tetrahydrofuran was heated to 60° C. and stirred for 12 hours to obtain a white precipitate. The resulting white precipit...
Embodiment 3
[0095] Prepare the DOPO derivative 3# of structural formula (3), there are 3 DOPO residues in this DOPO derivative:
[0096]
[0097] The synthetic route of DOPO derivative 3# is as follows:
[0098]
[0099]
[0100]
[0101] The synthesis method of DOPO derivative 3# is as follows:
[0102] Step 1 Synthesis of DOPO-DICY
[0103] Add DOPO and dicyandiamide (DICY) into the reaction vessel at a molar ratio of 1:1, heat to 175°C and react for 6 hours to obtain DOPO-DICY;
[0104] Step 2 prepares the Mannich type base containing DOPO
[0105] Add 0.01 mol of DOPO-DICY, 0.02 mol of p-hydroxybenzaldehyde and 100 ml of THF into a three-necked flask, blow nitrogen into it, and react at 50°C for 6 hours, and solids precipitate out. The obtained solid was filtered, washed with THF, and then dried in a vacuum oven to obtain Schiff base 3 containing an aromatic group.
[0106] The aromatic group-containing Schiff base 3 and DOPO were added to the three-necked flask accord...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com