Preparation method of celecoxib impurity B
A technology for celecoxib and impurities, which is applied in the field of preparation of impurities B in the celecoxib process, and can solve the problems of increasing production costs, being unsuitable for practical production applications, and having low yields.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: Synthesis of 4,4,4-trifluoro-1-(4-methylphenyl)butane-1,3-dione
[0034] Under nitrogen protection, methyl tert-butyl ether (3.0 L) and 20% by weight sodium ethoxide ethanol solution (3.55 kg, 10.43 mol) were added respectively, and the nitrogen was replaced three times. Control the temperature not to exceed 25±5°C, stir mechanically, and add ethyl trifluoroacetate (3) (1.27kg, 8.94mol) and p-methylacetophenone (4) (1.0kg, 7.45mol) in batches in sequence. Under nitrogen protection, the reaction temperature was controlled at 25±5° C., and mechanically stirred for 24 hours. It was detected by TLC that the raw materials disappeared and the reaction was completed (developing solvent: petroleum ether / ethyl acetate, volume ratio: 1 / 1).
[0035] Stop the reaction, add 5% by weight hydrochloric acid solution (6.5L) to the reaction solution to adjust the pH to 7.0-8.0, let stand for 15min, separate layers, collect the organic phase, extract the aqueous phase with eth...
Embodiment 2
[0036] Embodiment 2: Preparation of celecoxib impurity B
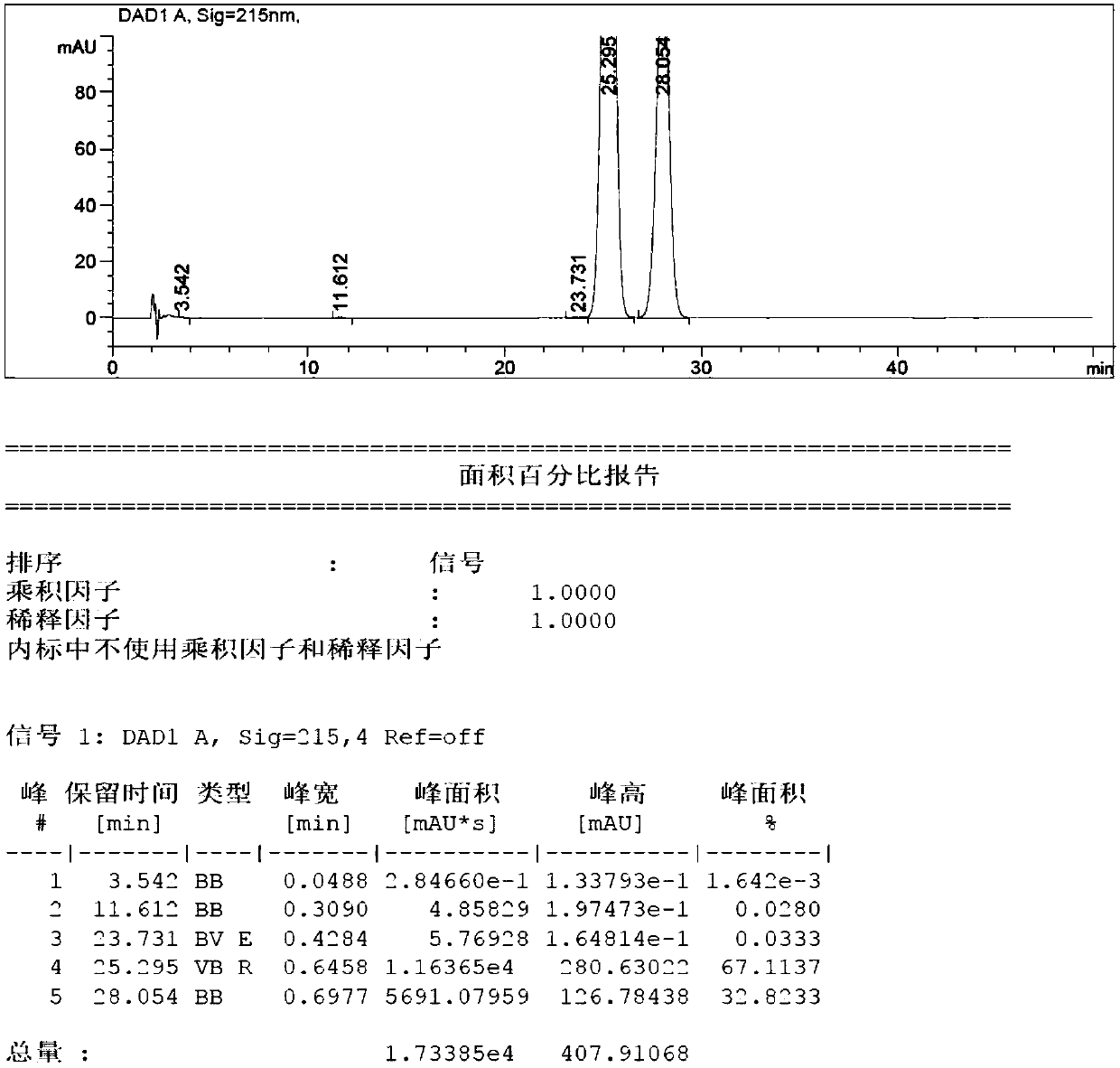
[0037] Add absolute ethanol (19.2L), purified water (4.8L) and 4,4,4-trifluoro-1-(4-methylphenyl)butane-1,3-dione (1.6 kg, 6.84mol), dissolved completely, added 4-hydrazinobenzenesulfonamide hydrochloride (1.61kg, 7.18mol), stirred mechanically, raised the temperature to reflux, reacted for 20h, compound 1 disappeared as detected by HPLC, and stopped heating. The reaction mixture was cooled and crystallized. When the temperature reached 20°C, the temperature was kept and stirred for 3h. Centrifuge, wash the filter cake with a small amount of mixed solvent (absolute ethanol / water: 1 / 1), collect the solid wet product, and dry the wet product at 45°C for 16 hours to obtain the crude product. The content of impurity B in the crude product is 32.8% as detected by HPLC (attached figure 1 ).
Embodiment 3
[0038] Embodiment 3: Preparation of celecoxib impurity B
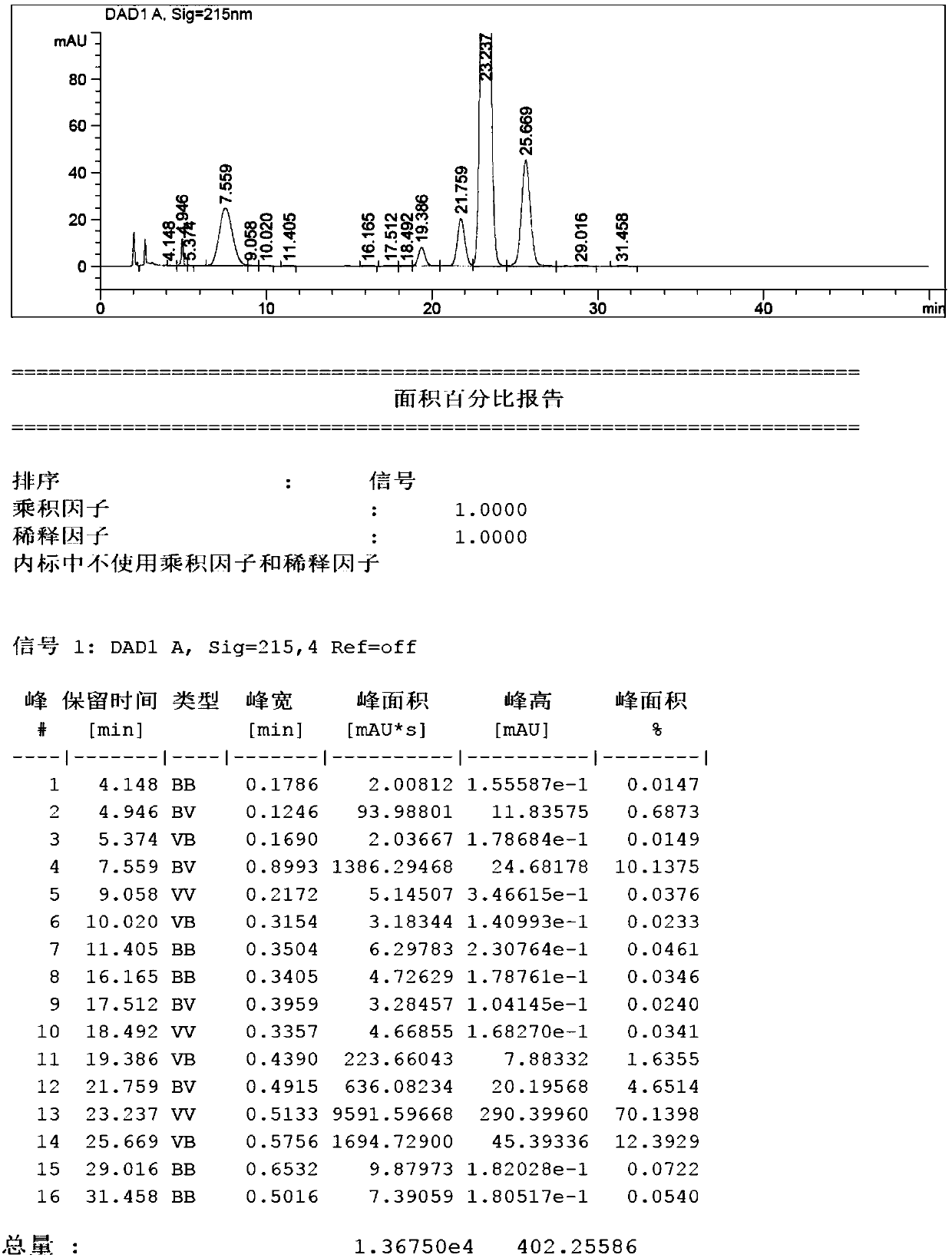
[0039]Take 10g of 4,4,4-trifluoro-1-(4-methylphenyl)butane-1,3-dione, add ethanol (120mL) to dissolve completely, measure its pH value to 7~8, then add Purified water (30mL) and 4-hydrazinobenzenesulfonamide hydrochloride (10.2g, 46.0mmol) were stirred, heated up, and refluxed for 20h. After sampling, HPLC detected that the content of impurity B reached 26.1%. The reaction was stopped, and concentrated to dryness under reduced pressure. .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com