Hydrated potassium borate and hydrated potassium borate nonlinear optical crystal and preparation method and application
A hydrated potassium borate and nonlinear optics technology, which is applied in nonlinear optics, chemical instruments and methods, optics, etc., can solve the problems of high price, long growth period, and easy deliquescence of crystals, and achieve simple preparation and short growth period Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
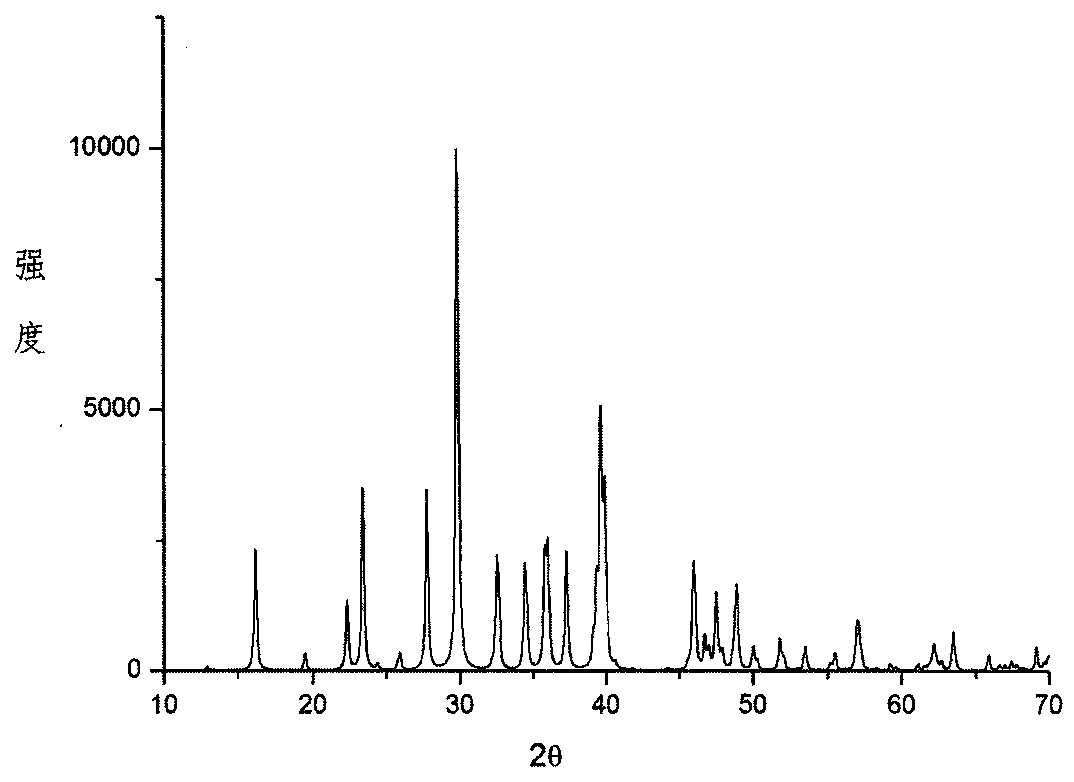
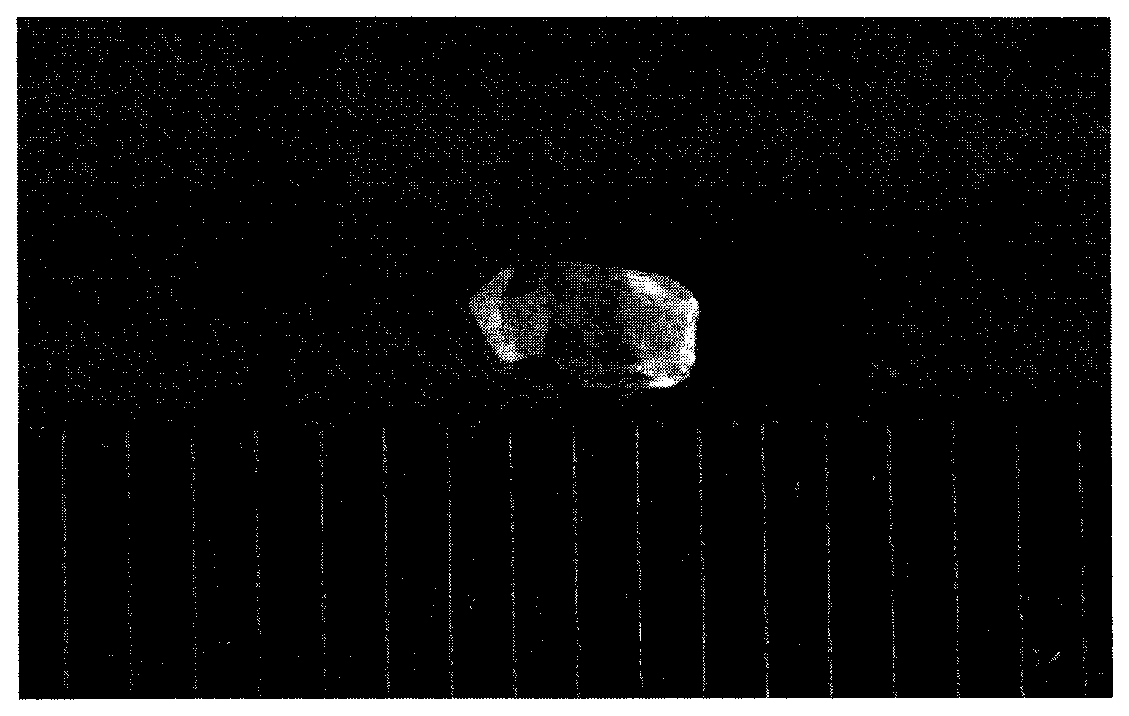
[0040] According to the chemical reaction formula KOH+H 3 BO 3 +H 2 O→K 3 B 3 o 4 (OH) 4 2H 2 O+H 2 O prepares transparent K 3 B 3 o 4 (OH) 4 2H 2 O crystals;
[0041] Weigh KOH solid and H at a molar ratio of 1:1 3 BO 3 Add the powder to the polytetrafluoroethylene lining of a 25mL autoclave, add 1mL of deionized water, and treat the incompletely dissolved mixture in an ultrasonic wave at a temperature of 30°C for 60 minutes to make it fully mixed;
[0042] Take out the polytetrafluoroethylene liner, cool naturally to room temperature, add KOH to adjust the pH value to 11;
[0043] Compress the PTFE-lined lid, put it into a corresponding clean, pollution-free stainless steel high-pressure reactor, and tighten the piston;
[0044] Put the reaction kettle into a constant temperature box, raise the temperature to 180°C at a rate of 30°C / h, keep the temperature for 6 days, and then lower it to room temperature at a rate of 20°C / day;
[0045] Open the reaction ket...
Embodiment 2
[0047] With the chemical reaction formula K 2 CO 3 +H 3 BO 3 +H 2 O→K 3 B 3 o 4 (OH) 4 2H 2 O+CO 2 ↑+H 2 O Transparent K 3 B 3 o 4 (OH) 4 2H 2 O crystals;
[0048] Weigh K with a molar ratio of 1:2 2 CO 3 Solid and H 3 BO 3 Add the powder to the polytetrafluoroethylene lining of a 40mL autoclave, add 5mL of deionized water, and ultrasonically treat the incompletely dissolved mixture at a temperature of 40°C for 60 minutes to make it fully mixed;
[0049] Then the polytetrafluoroethylene liner was taken out, cooled to room temperature naturally, and KOH was added to adjust the pH value to 10;
[0050] Compress the PTFE-lined lid, put it into a corresponding clean, pollution-free stainless steel high-pressure reactor, and tighten the piston;
[0051] Put the reaction kettle into a constant temperature box, raise the temperature to 170°C at a rate of 50°C / h, keep the temperature for 3 days, and then lower it to room temperature at a rate of 30°C / day;
[0052] ...
Embodiment 3
[0054] According to the chemical reaction formula KOH+B 2 o 3 +H 2 O→K 3 B 3 o 4 (OH) 4 2H 2 O+H 2 O Transparent K 3 B 3 o 4 (OH) 4 2H 2 O crystals;
[0055] Weigh KOH solid and B with a molar ratio of 1:1 2 o 3 Add the powder to the polytetrafluoroethylene lining of a 35mL autoclave, add 2mL of deionized water, and treat the incompletely dissolved mixture at a temperature of 60°C in ultrasonic waves for 60 minutes to make it fully mixed;
[0056] Then the polytetrafluoroethylene liner was taken out, cooled to room temperature naturally, and KOH was added to adjust the pH value to 11;
[0057] Compress the PTFE-lined lid, put it into a corresponding clean, pollution-free stainless steel high-pressure reactor, and tighten the piston;
[0058] Put the reaction kettle into a constant temperature box, raise the temperature to 200°C at a temperature increase rate of 40°C / h, keep the temperature for 10 days, and then lower it to room temperature at a temperature drop...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com