A kind of detection method of quinazoline-7-ether compound and its related substances
A technology of ether compounds and related substances, applied in the field of chemical analysis, can solve problems such as high structural similarity, low chromatographic peak separation, difficult detection of quinazoline-7-ether compounds, and achieve high peak symmetry and high The effect of system adaptability and high precision
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
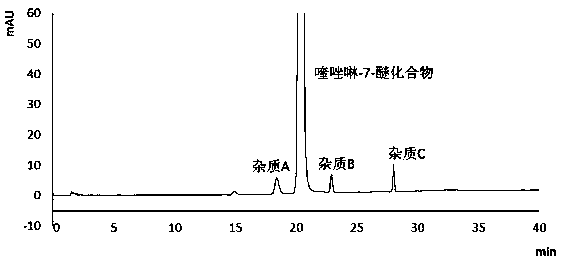
[0070] Determination of related substances in quinazoline-7-ether compound products
[0071] Instrument: Agilent High Performance Liquid Chromatograph
[0072] Column: Gemini C18 (4.6×150mm, 5μm)
[0073] Mobile phase A: 10mM ammonium acetate buffer solution (adjust the pH value to 5.0 with phosphoric acid)
[0074] Mobile phase B: methanol
[0075] Mobile Phase C: Acetonitrile
[0076] Detection wavelength: 253nm
[0077] Flow rate: 1.0ml / min
[0078] Injection volume: 20μl
[0079] Column temperature: 30℃
[0080] Dilution solvent: 10mM ammonium acetate buffer solution (adjust the pH to 5.0 with phosphoric acid), methanol and acetonitrile in a volume ratio of 55:30:15.
[0081] The elution procedure is shown in Table 1:
[0082] Table 1 Elution procedure
[0083] Time (min) Mobile phase A (%) Mobile phase B (%) Mobile phase C (%) 0553015 1544560 2035650 2510900
[0084] Test solution: Take 25 mg of the quinazoline-7-ether compound sample, put it in a 25ml measuring flask, add a diluent so...
Embodiment 2
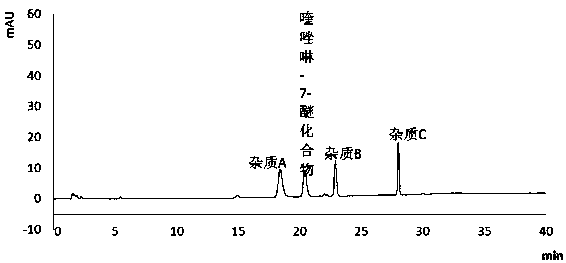
[0093] System adaptability detection of detection method
[0094] 1. Prepare the solution
[0095] 1) Dilute solution: mix 10mM ammonium acetate buffer solution (adjust the pH value to 5.0 with phosphoric acid), methanol and acetonitrile according to the volume ratio of 55:30:15 and mix well;
[0096] 2) Blank solution: dilute solution;
[0097] 3). Impurity stock solution: take 5mg of impurity A (structure compound of formula (II)), impurity B (structure compound of formula (III)), and impurity C (structure compound of formula (IV)), accurately weigh and place them in the same Dissolve and dilute to the mark with a blank solution in a 20ml measuring flask, shake well;
[0098] 4) Resolution solution: Take about 25mg of quinazoline-7-ether compound, put it in a 25ml measuring flask, add 0.1ml of impurity stock solution, dissolve it with the diluted solution and dilute to the mark, shake it well.
[0099] 2. Chromatographic detection:
[0100] Inject the above blank solution and resolutio...
Embodiment 3
[0106] Specificity testing of testing methods
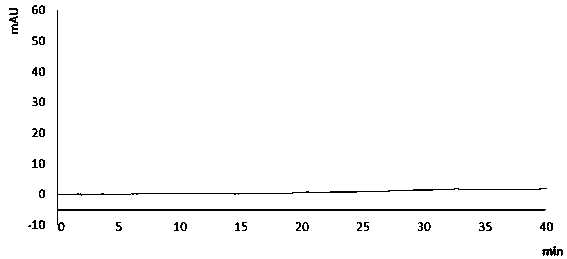
[0107] The strong degradation test is to accelerate the destruction of quinazoline-7-ether compounds under strong conditions, such as strong acid, strong alkali, strong oxidation, high temperature, and strong light. The purpose is to investigate the degradation products and The separation of main peaks and known impurities is compared with the amount of impurities produced and the reduction of main components to evaluate the effectiveness and applicability of the analytical method. At the same time, the DAD detector is used to check the peak purity: in the spectrum obtained from the degradation test, when the purity factor of the main component is greater than the threshold, it meets the requirements, that is, the main peak does not contain other unknown impurities.
[0108] According to the chromatographic conditions of Example 1, the samples treated with different strong degradation conditions were tested by high performance liquid ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com