Method for detecting effective components in asthma pellets
A detection method and technology of active ingredients, which is applied in the field of detection of active ingredients in asthma granules, can solve the problems of excipient interference, complex components, and inability to effectively quantify or qualitatively detect the active ingredients of asthma granules, and achieve good accuracy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Embodiment 1 qualitative detection
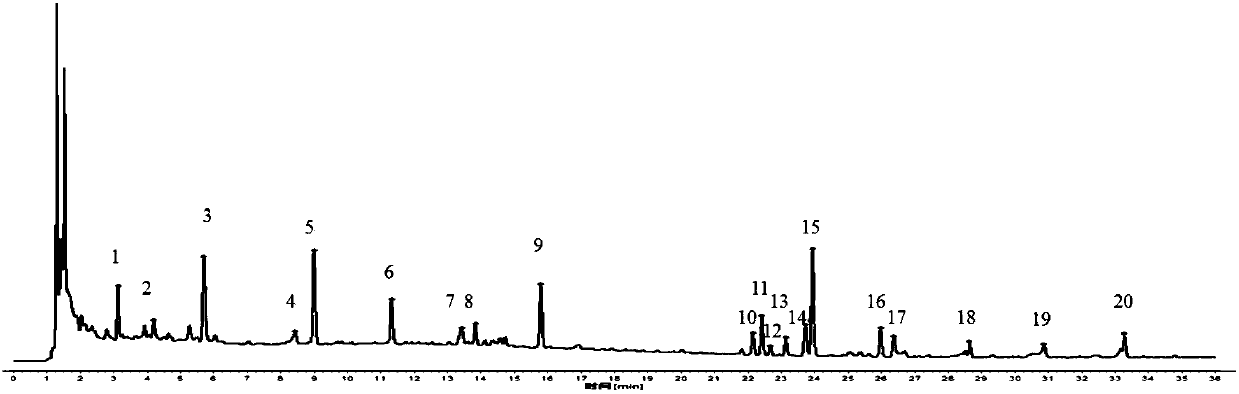
[0058] 1 Chromatographic conditions
[0059] The chromatographic column is Kromasil 100-3.5C18 (4.6X150mm, 3.5μm), and the mobile phase is methanol (A)-acetonitrile-(B)-water (C). The elution program is: 0~2min, 15%A; 2~16min , 15%~54%A, 16~30min, 54%~72%A; 30~36min, 72%~98%A; 0~36min, 2%B; column temperature: 40℃; flow rate 1.0mL·min -1 ; Detection wavelength: 210nm (laetrilein, magnolanin), 240nm (morroniside, loganin, acteoside, 5-O-visamigoside, schisandrin A), injection volume 5 μL .
[0060] 2 Selection of reference objects
[0061] Select 5-O-visamigoside with moderate retention time and better separation as a reference.
[0062] 3 Preparation of the test solution
[0063] Take the asthma granules (batch number: 141101) under the item of difference in loading amount, mix well, take an appropriate amount, grind finely, take about 1.0 g, weigh it accurately, put it in a stoppered Erlenmeyer flask, add 25 mL of 50% methanol ...
Embodiment 2
[0078] Embodiment 2 quantitative detection
[0079] 1 Chromatographic conditions
[0080] The chromatographic column is Kromasil 100-3.5C18 (4.6×150mm, 3.5μm), and the mobile phase is methanol (A)-acetonitrile-(B)-water (C). The elution program is: 0~2min, 15%A; 2~ 16min, 15%~54%A, 16~28min, 54%~72%A; 0~28min, 2%B; column temperature: 40℃; flow rate 1.0mL·min-1; detection wavelength: 210nm (amygdalin , magnolanin), 240nm (morroniside, loganin, acteoside, 5-O-visamigoside, schisandrin A), injection volume 5 μL.
[0081] 2. Preparation of reference substance stock solution
[0082] Precisely weigh an appropriate amount of morroniside, amygdalin, loganin, acteoside, 5-O-visamigoside, magnolanin and schisandrin A reference substance, and add 50% methanol to make a mixed standard Stock solution (morroniside content, amygdalin, loganin content 640.93 μg·mL -1 , Actinoside content 171.78μg·mL -1 , 5-O-Visamidol glycoside content 283.62μg·mL -1 , magnolanin, schisandrol A conten...
Embodiment 3
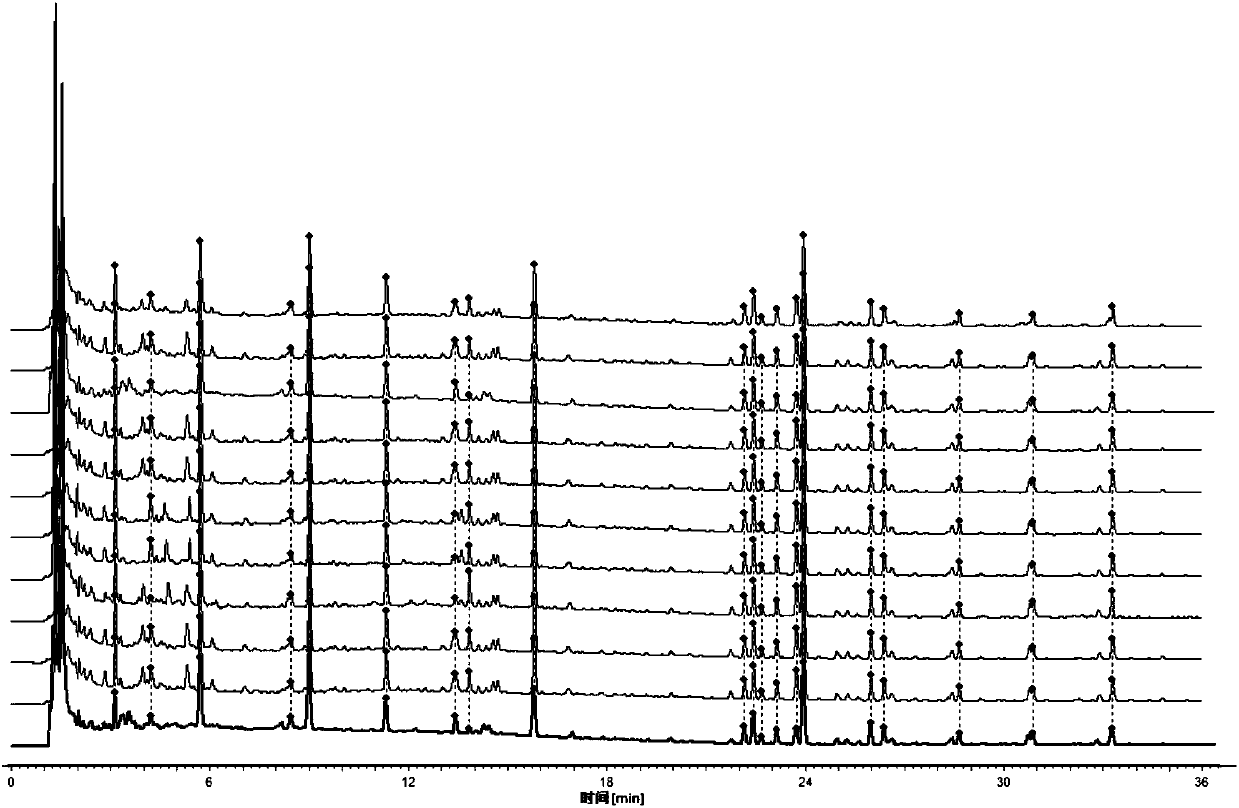
[0114] Embodiment 3 missing square detection
[0115] Five control medicines lacking bitter almond, Magnolia magnolia, Cornus officinalis, Fangfeng and Schisandra chinensis were prepared respectively. The reference substance solution prepared in Example 2 and the test sample (asthma granules, lot number 141101.) were used as contrasts to perform chromatographic detection and observe the difference in chromatograms.
[0116] 1 Chromatographic conditions
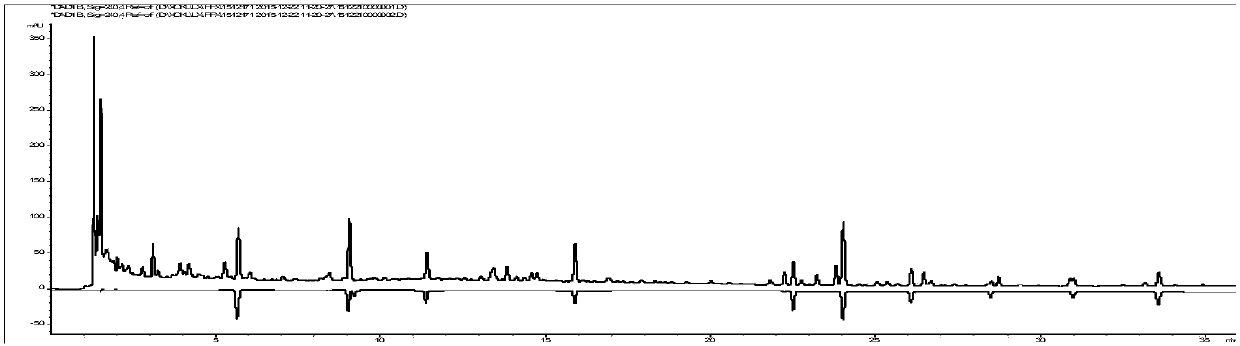
[0117] The chromatographic column is Kromasil 100-3.5C18 (4.6×150mm, 3.5μm), and the mobile phase is methanol (A)-acetonitrile-(B)-water (C). The elution program is: 0~2min, 15%A; 2~ 16min, 15%~54%A, 16~28min, 54%~72%A; 0~28min, 2%B; column temperature: 40℃; flow rate 1.0mL·min-1; detection wavelength: 210nm (amygdalin , magnolanin), 240nm (morroniside, loganin, acteoside, 5-O-visamigoside, schisandrin A), injection volume 5 μL. Test results such as Figure 4~5 .
[0118]It can be seen that according to the method provide...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com