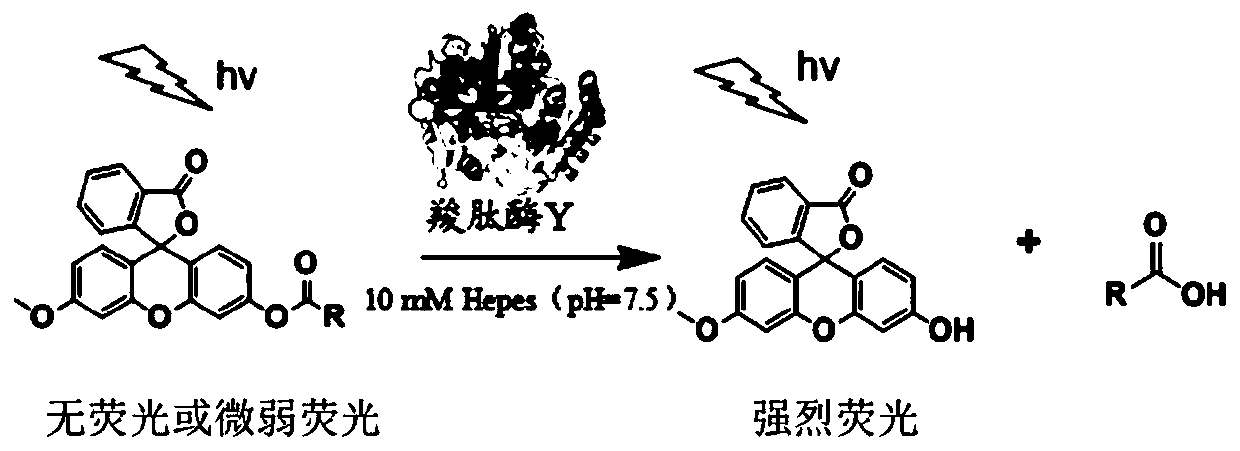
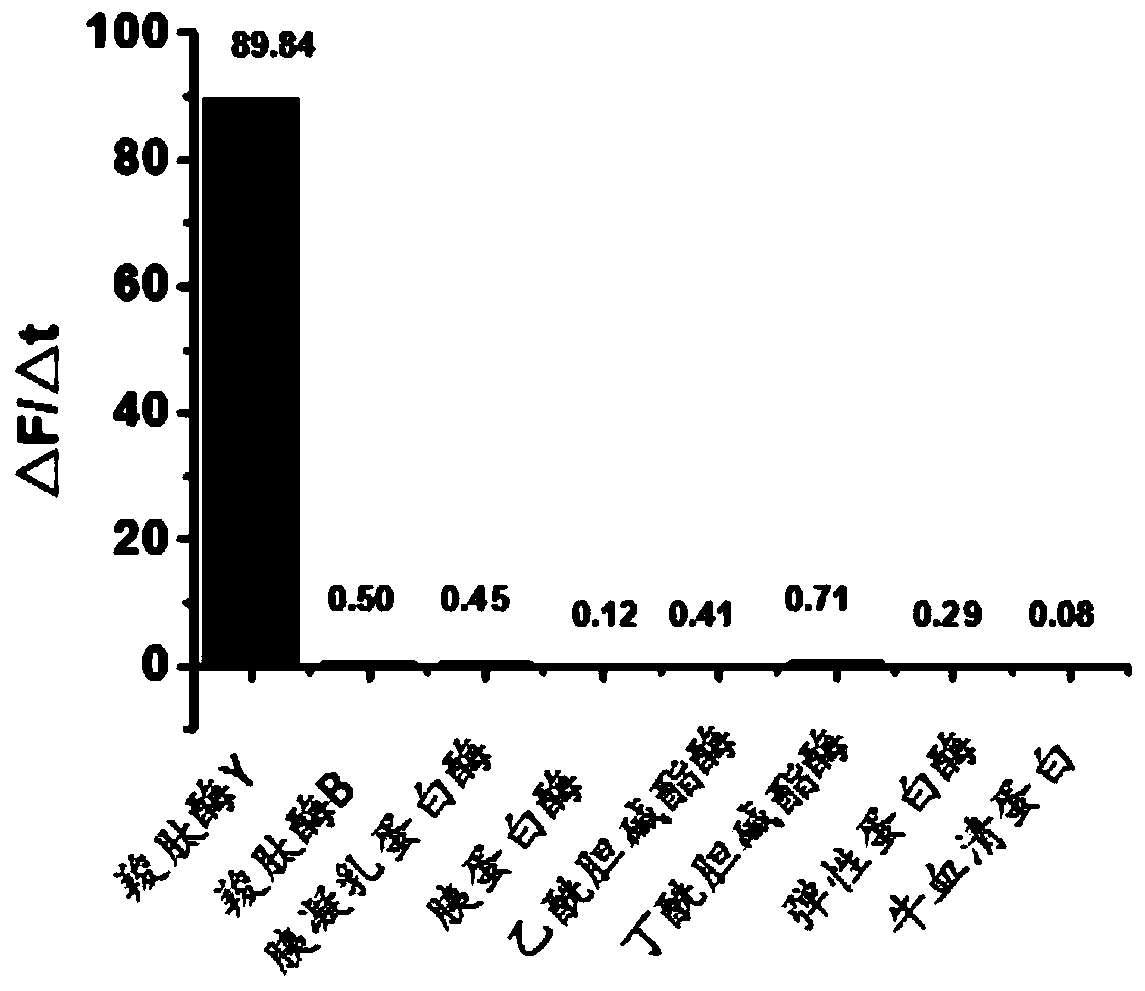
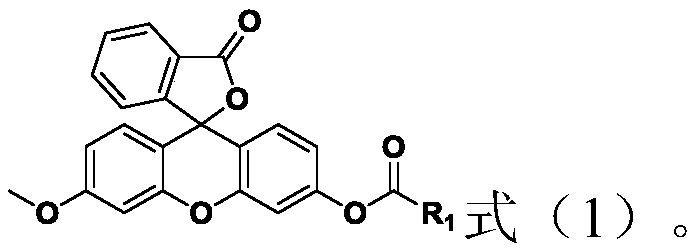
Methoxyfluorescein derivative and its preparation method, carboxypeptidase y detection method and detection kit
A detection method, fluorescein technology, applied in the direction of fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of harsh storage and permission conditions, difficult peptide substrate synthesis, and difficult substrate synthesis, etc., to achieve High sensitivity and selectivity, low price, high sensitivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] In a second aspect, the present invention also provides a method for preparing the above-mentioned methoxyfluorescein derivatives, the method comprising: in an organic solvent under the conditions of a nucleophilic reaction and the presence of an acid-binding agent, the The structure compound is contacted with one of acid chloride or acid anhydride;
[0034]
[0035] Wherein, the general formula of the acid chloride is R'-C(=O)Cl, R' has the same selection as R; that is, R' is a C1-C10 alkyl or cycloalkyl group; preferably, R' It is an alkyl group of C2-C6; more preferably, R' is selected from one of ethyl, propyl, butyl, pentyl, hexyl, cyclopropyl, cyclobutyl, cyclopentyl and cyclohexyl; more Preferably, R' is selected from one of ethyl, propyl, butyl and cyclohexyl, more preferably, R' is selected from ethyl, n-propyl, isopropyl, tert-butyl and cyclohexyl (that is, the acid chloride is selected from one of propionyl chloride, n-butyryl chloride, isobutyryl chlorid...
Embodiment 1
[0076] This example is used to illustrate the preparation method of the methoxyfluorescein derivatives of the present invention.
[0077] In an organic solvent (dichloromethane, 20mL), in the presence of an acid-binding agent (triethylamine, 2mmol), the compound represented by formula (2) (methoxyfluorescein, 1mmol) and acid chloride (trimethyl Acetyl chloride, 2 mmol) was maintained at 5°C for 120 min to obtain the contacted material. Then, the contacted material was washed with water (50 mL) and saturated saline solution (50 mL) successively to obtain the washed material. The organic phase in the washed material was separated and dried with anhydrous sodium sulfate, and the solvent was removed under reduced pressure to obtain a crude product, which was purified by a silica gel column to obtain 0.27 g of a white solid product.
[0078] The white solid product was characterized by NMR and HRMS, and the data are as follows. 1 H NMR (600MHz, DMSO-d 6 ): δ8.02(d, J=11.4Hz, 1H)...
Embodiment 2
[0082] This example is used to illustrate the preparation method of the methoxyfluorescein derivatives of the present invention.
[0083] The preparation of methoxyfluorescein derivatives was carried out according to the method of Example 1, except that cyclohexylformyl chloride was used as the acid chloride, and finally 0.31 g of white solid was obtained.
[0084] Detect and characterize this white solid product with NMR and HRMS, data: 1H NMR (600MHz, DMSO-d 6 ): δ8.05(d, J=7.2Hz, 1H), 7.81(t, J=7.2Hz, 1H), 7.75(t, J=7.2Hz, 1H), 7.33(d, J=7.2Hz, 1H ), 7.23(s, 1H), 6.96(s, 1H), 6.92-6.86(m, 2H), 6.74(q, J=8.8Hz, 2H), 3.82(s, 3H), 2.61(quintuplet, J= 9.6Hz,1H),1.99-1.97(m,2H),1.73-1.71(m,2H),1.51-1.46(m,2H),1.36-1.30(m,2H),1.25-1.22(m,2H) .HRMS calcd for [M+H] + :457.1646. Found: 457.1648..
[0085] This data completely agrees with the theoretical value of the compound shown in formula (1), proves that this product is the compound shown in formula (4).
[0086]
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com