Injecting carfilzomib and preparation method thereof
A carfilzomib and prescription technology, which is applied in the field of carfilzomib for injection and its preparation, to achieve the effects of reducing production costs, solving oxidation, and solving insoluble problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
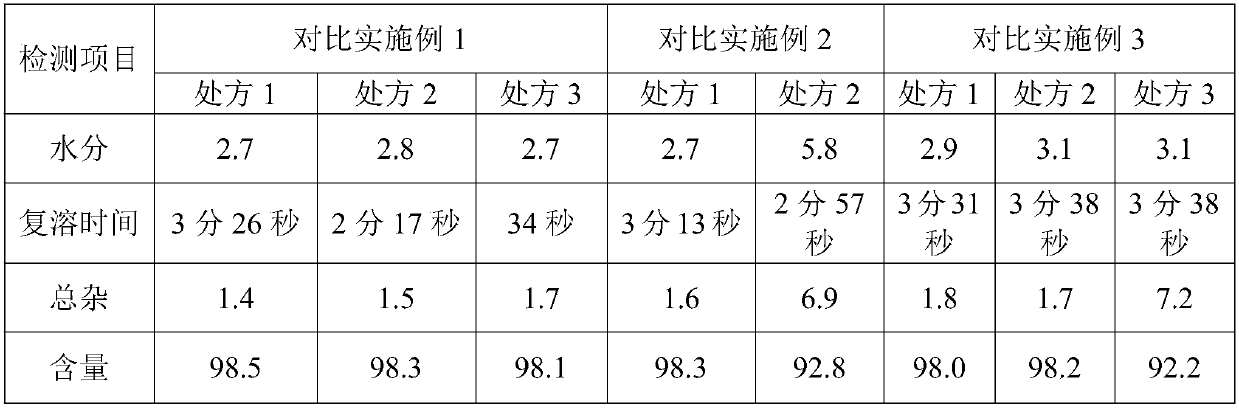
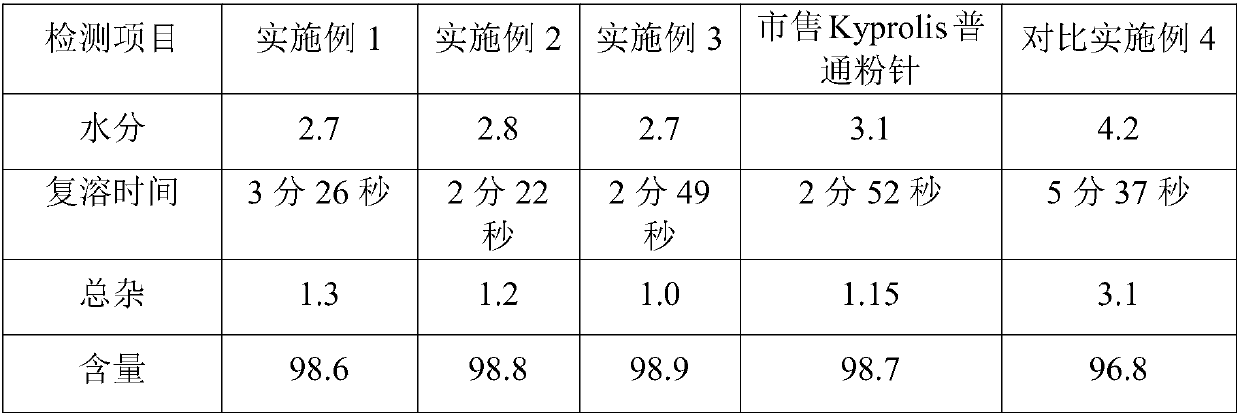
Embodiment 1
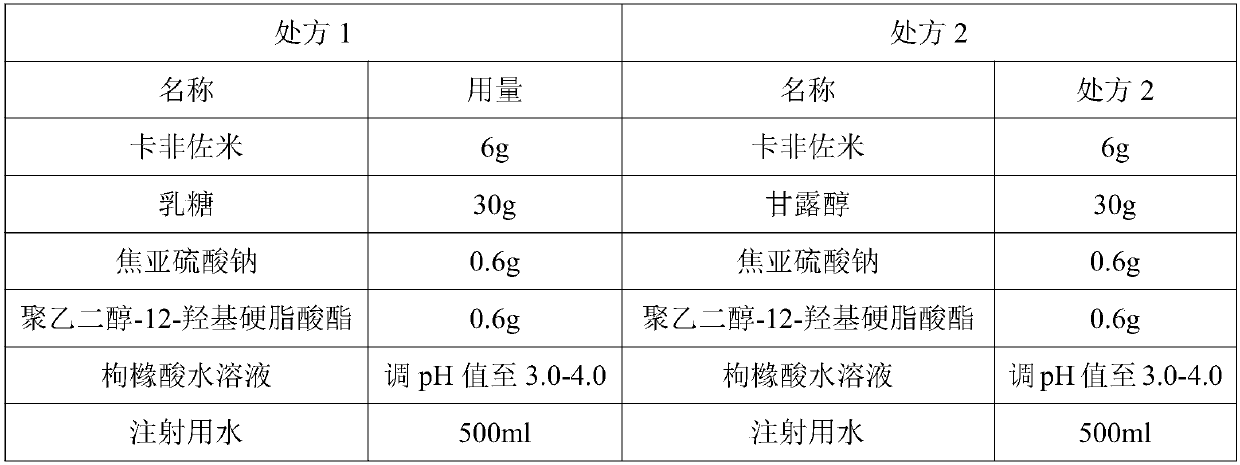
500ml
[0034] Preparation Process:
[0035] 1) Take 0.6g of solubilizer and 0.6g of antioxidant, add to 100ml of water for injection, bubbling nitrogen in the water for 15min, and stir;
[0036] 2) Take 6g of Carfilzomib, add it to the solution obtained in step 1) under the protection of nitrogen, and stir until the solution is clear;
[0037] 3) Take 30g of lactose, add 250ml of water for injection, stir to dissolve;
[0038] 4) Under nitrogen protection, add the solution obtained in step 2) to the solution obtained in step 3), and stir until the solution is clear;
[0039] 5) Add water for injection to 95% of the prescription and adjust the pH to 3.8;
[0040] 6) Add water for injection to 500ml, stir evenly to obtain the intermediate liquid, and retest the pH value of 3.8;
[0041] 7) The intermediate medicinal solution is press-filtered, sub-packaged, freeze-dried, stoppered, capped, packaged, and obtained carfilzomib freeze-dried powder injection. The freeze-drying process is as fol...
Embodiment 2
500ml
[0044] Preparation Process:
[0045] 1) Take 3g of solubilizer and 1.5g of antioxidant, add to 100ml of water for injection, bubbling nitrogen in the water for 15min, and stir;
[0046] 2) Take 6g of Carfilzomib, add it to the solution obtained in step 1) under the protection of nitrogen, and stir until the solution is clear;
[0047] 3) Take 40g of lactose, add 250ml of water for injection, stir to dissolve;
[0048] 4) Under nitrogen protection, add the solution obtained in step 2) to the solution obtained in step 3), and stir until the solution is clear;
[0049] 5) Add water for injection to 95% of the prescription and adjust the pH to 3.4;
[0050] 6) Add water for injection to 500ml, stir evenly to obtain the intermediate liquid, and retest the pH value of 3.5;
[0051] 7) The intermediate medicinal solution is press-filtered, sub-packaged, freeze-dried, stoppered, capped, packaged, and obtained carfilzomib freeze-dried powder injection. The freeze-drying process is as follo...
Embodiment 3
500ml
[0054] Preparation Process:
[0055] 1) Take 2g of solubilizer and 3g of antioxidants, add to 100ml of water for injection, bubbling nitrogen in the water for 15 minutes, and stir;
[0056] 2) Take 6g of Carfilzomib, add it to the solution obtained in step 1) under the protection of nitrogen, and stir until the solution is clear;
[0057] 3) Take 48g of lactose, add 250ml of water for injection, stir to dissolve;
[0058] 4) Under nitrogen protection, add the solution obtained in step 2) to the solution obtained in step 3), and stir until the solution is clear;
[0059] 5) Add water for injection to 95% of the prescription and adjust the pH to 3.7;
[0060] 6) Add water for injection to 500ml, stir evenly to obtain the intermediate liquid, and retest the pH value of 3.8;
[0061] 7) The intermediate medicinal solution is press-filtered, sub-packaged, freeze-dried, stoppered, capped, packaged, and obtained carfilzomib freeze-dried powder injection. The freeze-drying process is as f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com