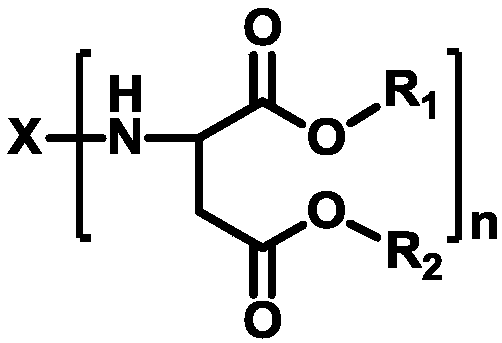
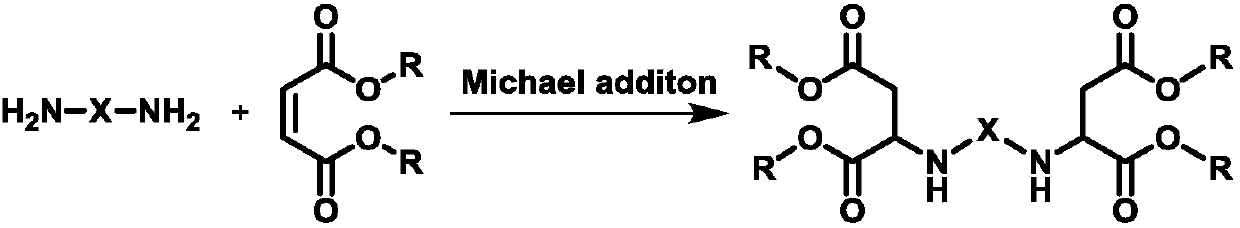
Preparation method of polyaspartic acid ester
A technology for aspartate and dibasic acid ester, which is applied in the field of preparation of polyaspartate, can solve problems such as adverse effects of downstream applications, unsatisfactory catalytic effect, etc., and achieves excellent chromaticity and easy removal. , the effect of high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] (1) 174.31g (1.5mol) 2-methylpentanediamine and 0.137g (1.5mmol) tetramethylammonium hydroxide catalyst are added to be equipped with mechanical stirring paddle, thermometer, constant pressure dropping funnel, connected with N 2 In the 1L three-necked flask of the gas line and bubbler, 516.54g (3.0mol) diethyl maleate was added in the constant pressure dropping funnel, and N was passed into the system. 2 For 20 minutes, the air in the system was replaced; diethyl maleate was slowly added dropwise under stirring at 25°C, and after the drop was completed, the temperature was raised to 40°C for 2 hours to stop the reaction.
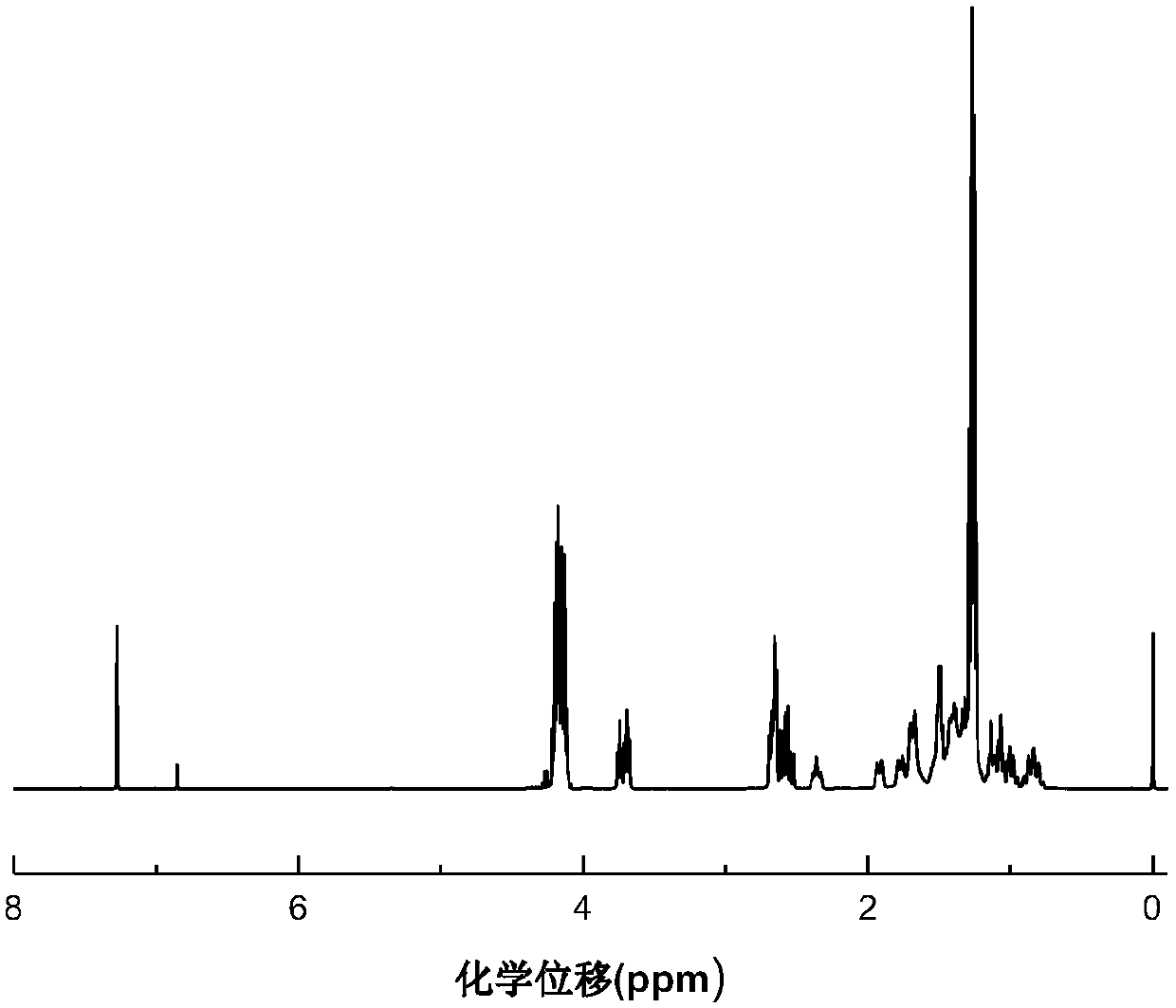
[0039] (2) Heating to 90°C, nitrogen flow, water pump extraction under 0.001MPa pressure for 20 minutes to remove tetramethylammonium hydroxide catalyst, and obtain polyaspartic acid ester PAE-1, its structure is shown in the following formula:
[0040]
[0041] (3) Characterization analysis results, diethyl maleate conversion rate 99.7%, Hazen chro...
Embodiment 2
[0043] (1) Add 174.31g (1.5mol) of 2-methylpentanediamine and 0.044g (0.075mmol) of 25% tetraethylammonium hydroxide aqueous solution and add a mechanical stirring paddle, a thermometer, a constant pressure dropping funnel, and a N 2 In the 1L three-necked flask of the gas line and bubbler, 516.54g (3.0 mol) diethyl maleate was added to the constant pressure dropping funnel, and N was introduced into the system. 2 After 10 minutes, the air in the system was replaced; diethyl maleate was slowly added dropwise under the condition of stirring at 10°C, and after the drop was completed, the temperature was raised to 30°C for 6 hours to stop the reaction.
[0044] (2) Heating to 130°C, nitrogen flow, water pump extraction under 0.02MPa pressure for 40 minutes to remove tetraethylammonium hydroxide catalyst and moisture, and obtain polyaspartic acid ester PAE-1.
[0045] (3) Characterization analysis results, diethyl maleate conversion rate 99%, Hazen chromaticity 28, gel time 4min...
Embodiment 3
[0047] (1) 174.31g (1.5mol) 2-methylpentanediamine and 0.685g (7.5mmol) tetramethylammonium hydroxide catalyst are added to be equipped with mechanical stirring paddle, thermometer, constant pressure dropping funnel, connected with N 2 In the 1L three-necked flask of the gas line and bubbler, 516.54g (3.0mol) diethyl fumarate was added in the constant pressure dropping funnel, and N was passed into the system. 2 After 15 minutes, the air in the system was replaced; diethyl fumarate was slowly added dropwise under stirring at 40°C, and after the drop was completed, the temperature was raised to 70°C for 1 hour to stop the reaction.
[0048] (2) Heating to 120°C, nitrogen flow, water pump extraction under 0.01MPa pressure for 30min to remove tetramethylammonium hydroxide catalyst, and obtain polyaspartic acid ester PAE-1.
[0049] (3) Characterization and analysis results, the conversion rate of diethyl fumarate is 98.5%, the Hazen color is 29, the gel time is 4min, X is 2-meth...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Gel time | aaaaa | aaaaa |
| Gel time | aaaaa | aaaaa |
| Gel time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com