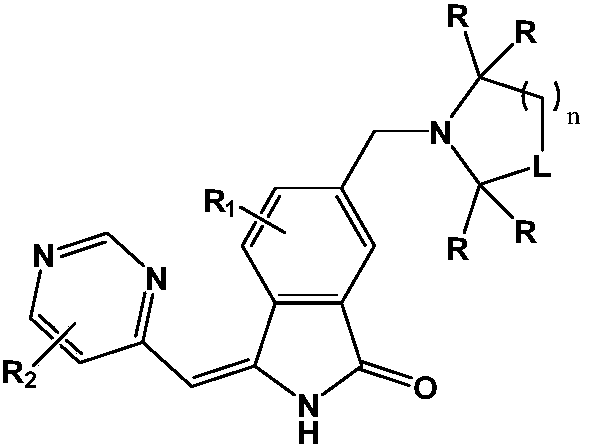
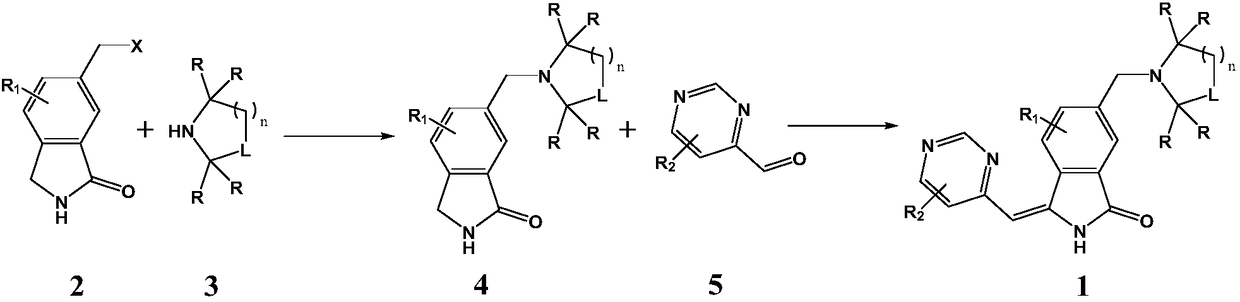
A kind of medicine for treating hepatitis B and its preparation method
A pharmacy and compound technology, applied in the field of medicine, can solve the problems of poor curative effect, easy recurrence of drug withdrawal, drug resistance, etc., and achieve the effects of good inhibitory effect, excellent anti-hepatitis B virus activity, and low toxicity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
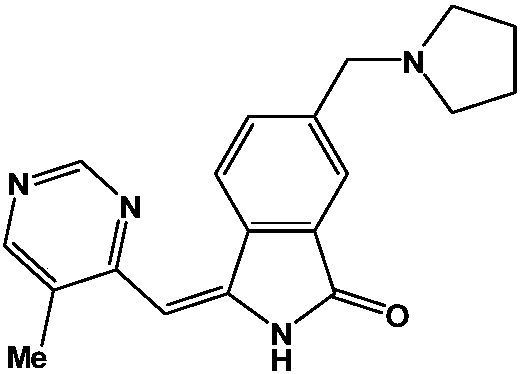
[0047] Example 1: (E)-3-((5-methylpyrimidin-4-yl)methylene)-6-(pyrrolidin-1-ylmethyl)isoindol-1-one (MIDO-1 )Synthesis
[0048]
[0049] Step 1: Mix 3.71 g of 6-(bromomethyl)isoindol-1-one, 1.42 g of tetrahydropyrrole, and 7.31 g of cesium carbonate in 50 mL of THF under a nitrogen atmosphere at -5°C. The reaction solution was warmed to room temperature and stirred overnight. The resulting solution was diluted with 200 mL water and then extracted with 3×100 mL ethyl acetate. The combined organic layer was washed once with 100 mL water and 100 mL brine each and dried over anhydrous calcium chloride. The solvent was removed by rotary evaporation, and the residue was purified by silica gel column chromatography (ethyl acetate: petroleum ether = 1:5) to obtain 3.17 g of intermediate 6-(pyrrolidin-1-ylmethyl)isoindol-1-one , The yield is 89.0%.
[0050] ESI-MS: 217.13[M+H] +
[0051] Step 2: Dissolve 1.46g of 5-methylpyrimidine-4-carbaldehyde in 150ml of absolute ethanol, then add 2....
Embodiment 2
[0055] Example 2: (E)-3-((6-hydroxypyrimidin-4-yl)methylene)-6-(piperazin-1-ylmethyl)isoindol-1-one (MIDO-2) Synthesis
[0056]
[0057] Step 1: Mix 3.71 g of 6-(bromomethyl)isoindol-1-one, 1.72 g of piperazine, and 7.31 g of cesium carbonate in 80 mL of THF at -5°C under a nitrogen atmosphere. The reaction solution was warmed to room temperature and stirred overnight. The resulting solution was diluted with 200 mL water and then extracted with 3×100 mL ethyl acetate. The combined organic layer was washed once with 100 mL water and 100 mL brine each and dried over anhydrous calcium chloride. The solvent was removed by rotary evaporation, and the residue was purified by silica gel column chromatography (ethyl acetate:n-hexane=1:3) to obtain 3.33 g of intermediate 6-(piperazin-1-ylmethyl)isoindol-1-one , The yield is 87.3%.
[0058] ESI-MS: 232.14[M+H] +
[0059] Step 2: Dissolve 1.48g of 6-hydroxypyrimidine-4-carbaldehyde in 100ml of absolute ethanol, then add 2.32g of 6-(piperaz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com