Preparation method of asymmetric 1,3-diyne compound
An alkyne compound and asymmetric technology, which is applied in the field of preparation of asymmetric 1,3-diyne compounds, can solve the problems of long reaction route, reduced yield, and increased difficulty of reaction, etc., and achieves simple operation and high reaction efficiency. The effect of fewer steps and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Synthesis of 1-chloro-4-(1,3-octadiynyl)benzene
[0029] Take a 25mL dry Schlenk bottle, add a stirring bar, and mix bromoalkene 1a (0.2mmol), DMSO (1mL, preliminarily dried through activated molecular sieves), cuprous iodide (CuI, 0.04mmol), sarcosine (Creatine ,0.08mmol), cesium carbonate (Cs 2 CO 3 , 0.8 mmol) were successively added into the Schlenk bottle under the protection of nitrogen, the temperature of the oil bath was 60°C, and the reaction was stirred for 4h. After the reaction, petroleum ether was extracted (3×10 mL), the organic layers were combined, rotary evaporated, and purified by preparative TLC to obtain the product (developer: petroleum ether, 60-90° C.), with a yield of 72%.
[0030]
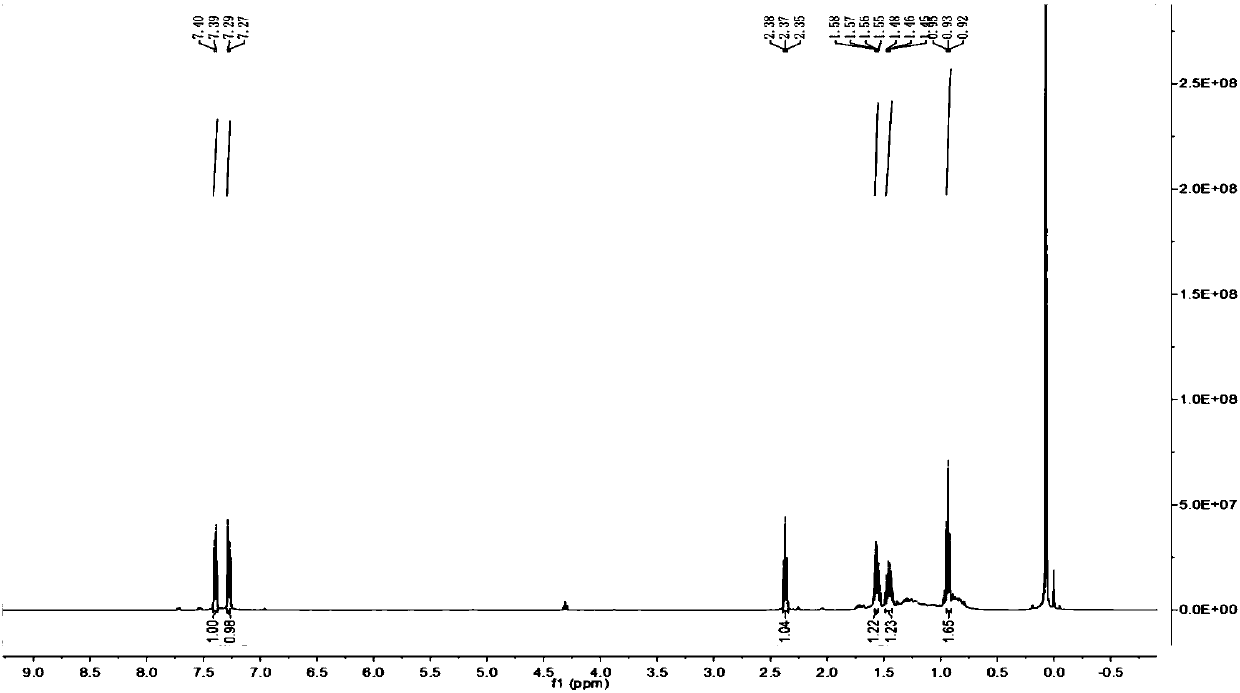
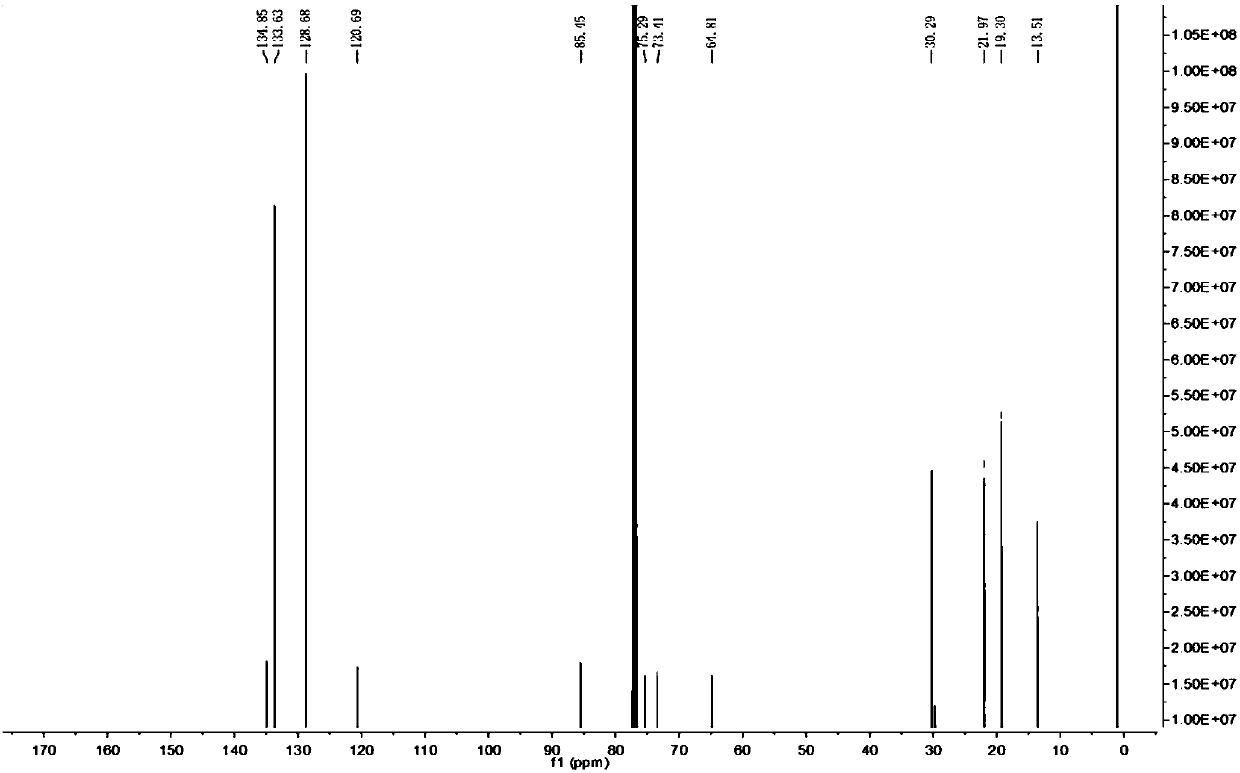
[0031] 1-Chloro-4-(1,3-octadiynyl)benzene
[0032] 1 H NMR (500MHz, CDCl3) δ7.40(d, J=8.4Hz, 2H), 7.28(d, J=8.4Hz, 2H), 2.37(t, J=7.0Hz, 2H), 1.61–1.51(m ,2H),1.45(m,2H),0.93(t,3H).
[0033] 13 C NMR (500MHz, CDCl3) δ134.85, 133.63, 128.69, 85.45, 75.29, 73...
Embodiment 2
[0037] Synthesis of 1-chloro-4-(1,3-heptadynyl)benzene
[0038] Take a 25 mL dry Schlenk bottle, add a stirring bar, and mix bromoalkene 2a (0.2 mmol), DMSO (1 mL, preliminarily dried through activated molecular sieves), cuprous iodide (CuI, 0.04 mmol), sarcosine (Creatine ,0.08mmol), cesium carbonate (Cs 2 CO 3 , 0.8 mmol) were successively added into the Schlenk bottle under the protection of nitrogen, the temperature of the oil bath was 60°C, and the reaction was stirred for 4h. After the reaction, petroleum ether was extracted (3×10 mL), the organic layers were combined, rotary evaporated, and purified by preparative TLC to obtain the product (developing solvent: petroleum ether, 60-90° C.), with a yield of 69%.
[0039]
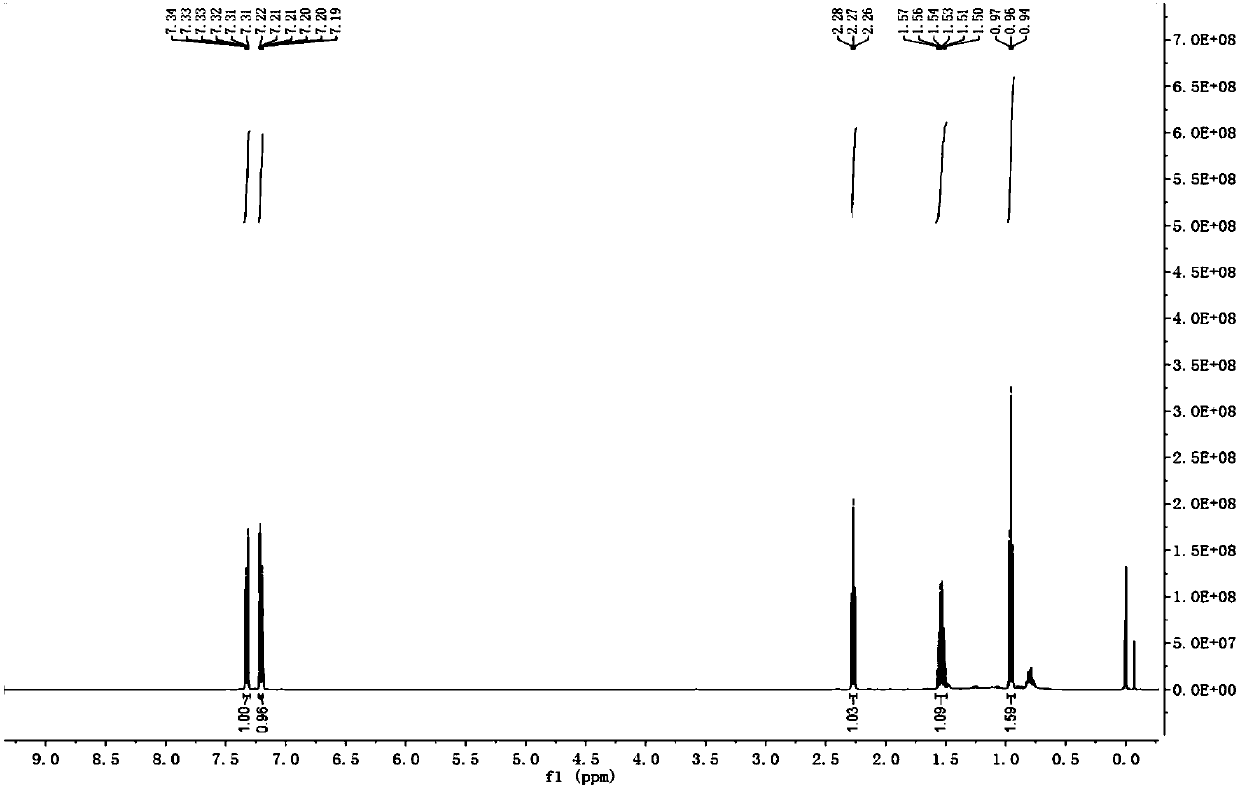
[0040] 1-Chloro-4-(1,3-heptadiynyl)benzene
[0041] 1 H NMR (500MHz, CDCl 3 )δ7.32(d, J=8.6Hz, 2H), 7.20(d, J=8.6Hz, 2H), 2.27 (t, J=7.0Hz, 2H), 1.58–1.47(m, 3H), 0.96( t,3H).
Embodiment 3
[0043] Synthesis of 4-bromo-2-fluoro-1-(1,3-heptadiynyl)benzene
[0044] Take a 25 mL dry Schlenk bottle, add a stirring bar, and mix bromoalkene 3a (0.2 mmol), DMSO (1 mL, preliminarily dried through activated molecular sieves), cuprous iodide (CuI, 0.04 mmol), sarcosine (Creatine ,0.08mmol), cesium carbonate (Cs 2 CO 3 , 0.8 mmol) were successively added into the Schlenk bottle under the protection of nitrogen, the temperature of the oil bath was 60°C, and the reaction was stirred for 4h. After the reaction, petroleum ether was extracted (3×10 mL), the organic layers were combined, rotary evaporated, and purified by preparative TLC to obtain the product (developing solvent: petroleum ether, 60-90° C.), with a yield of 47%.
[0045]
[0046] 4-Bromo-2-fluoro-1-(1,3-heptadiynyl)benzene
[0047] 1 H NMR (500MHz, CDCl3) δ7.27–7.23(m,1H),7.21–7.17(m,2H),2.28(t,J=7.0Hz,2H),1.60–1.49(m,2H),0.96( t,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com