Macrocyclic diterpene compounds in fruit of euphorbia sororia A. schrenk as well as preparation method of macrocyclic diterpene compounds and use of macrocyclic diterpene compounds in reversion of multidrug resistance
A technique for treating spurge, compounds, applied in the field of medicine, can solve the problems of drug resistance, increased drug efflux, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
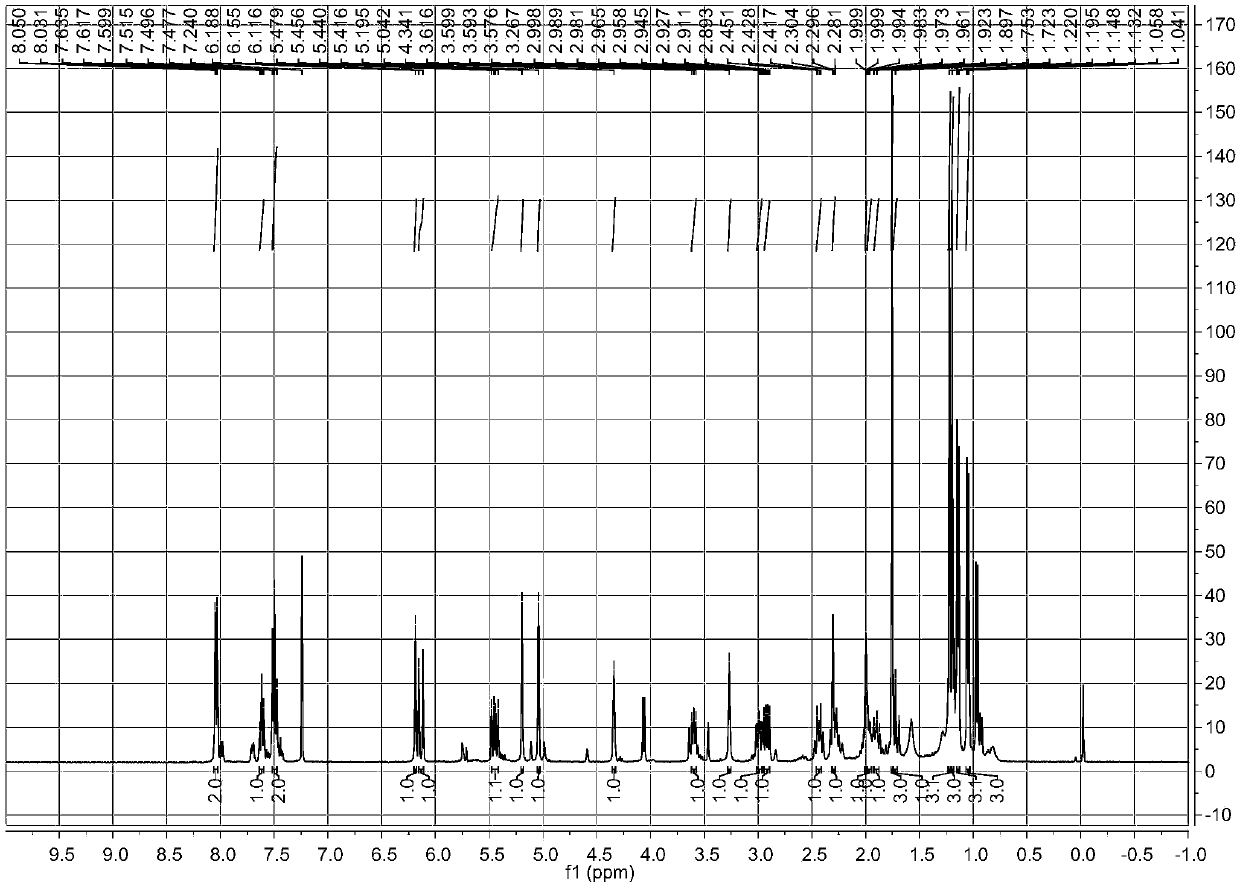
[0036] a. Take 10 kg of the fruit of Euphorbia euphorbia, crush it, and use 50 L of 50% ethanol-water solution to extract it by cold soaking at room temperature, and evaporate the solvent to dryness under reduced pressure to obtain the crude extract of Euphorbia ipsifolia;
[0037] b. Disperse the crude extract obtained in step a with ethanol, add petroleum ether for extraction, combine the ethanol layers and evaporate to dryness under reduced pressure to obtain the ethanol extract extract;
[0038] c, the ethanol extract extract obtained in step b is separated with a normal phase silica gel column, and gradient elution is carried out with petroleum ether-ethyl acetate with a volume ratio of 10:1-0:1, and the fraction is subjected to silica gel thin layer chromatography ( TLC) analysis, the same fractions were combined to obtain 6 fractions (F1-F6); fraction F4 was subjected to normal-phase silica gel column separation, and gradient elution was carried out with chloroform-aceto...
Embodiment 2
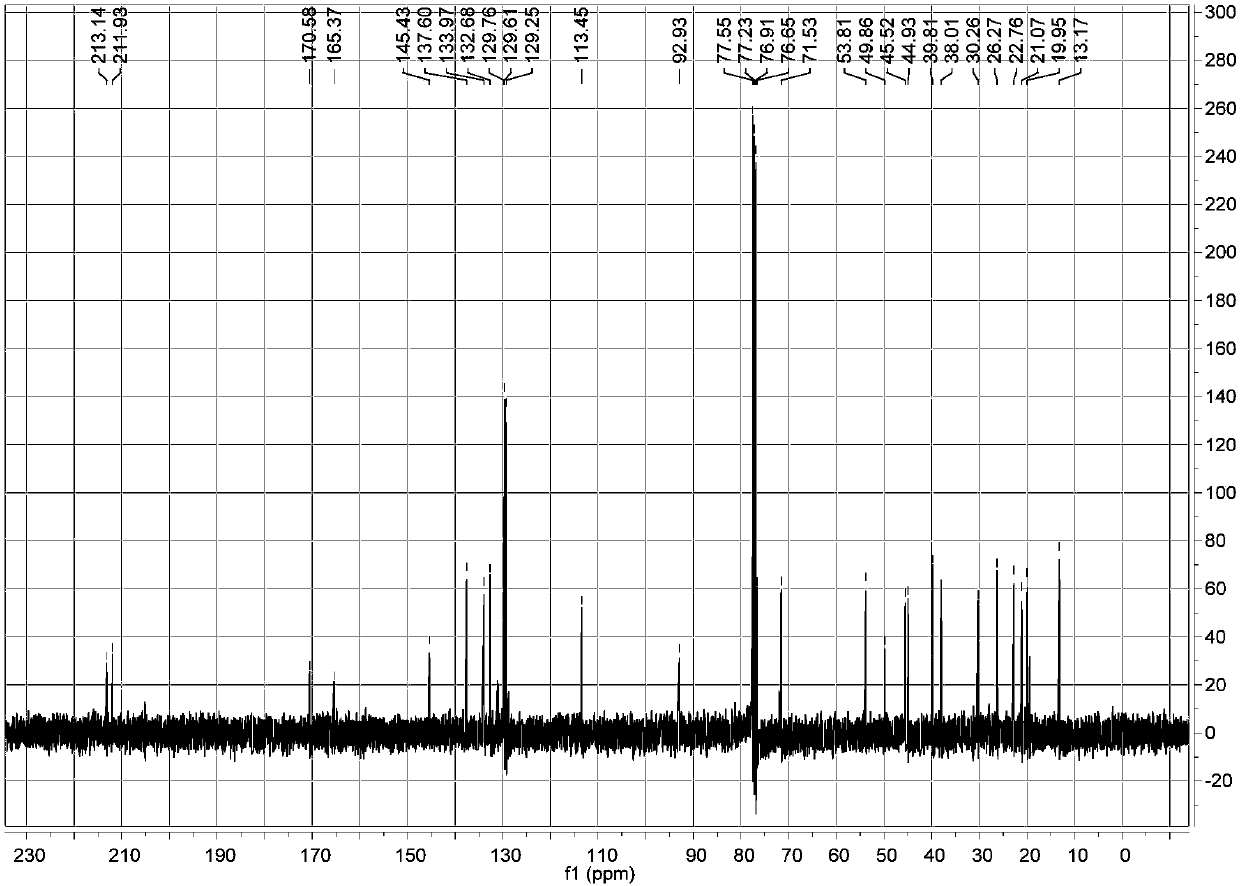
[0040] a. Take 10 kg of the fruit of Euphorbia euphorbia, crush it, and use 60 L of 99% ethanol-water solution to reflux extract at a temperature of 80° C., and evaporate the solvent to dryness under reduced pressure to obtain the crude extract of Euphorbia ipsifolia;
[0041] b. Disperse the crude extract obtained in step a with ethanol, add n-hexane for extraction, combine the ethanol layers and evaporate to dryness under reduced pressure to obtain the ethanol extract extract;
[0042] c, the ethanol extract extract obtained in step b is separated with a normal phase silica gel column, and gradient elution is carried out with n-hexane-ethyl acetate with a volume ratio of 10:1-0:1, and the fraction is subjected to silica gel thin layer chromatography ( TLC) analysis, the same fractions were combined to obtain 6 fractions (F1-F6); fraction F4 was subjected to normal phase silica gel column separation, and gradient elution was carried out with chloroform-methanol with a volume r...
Embodiment 3
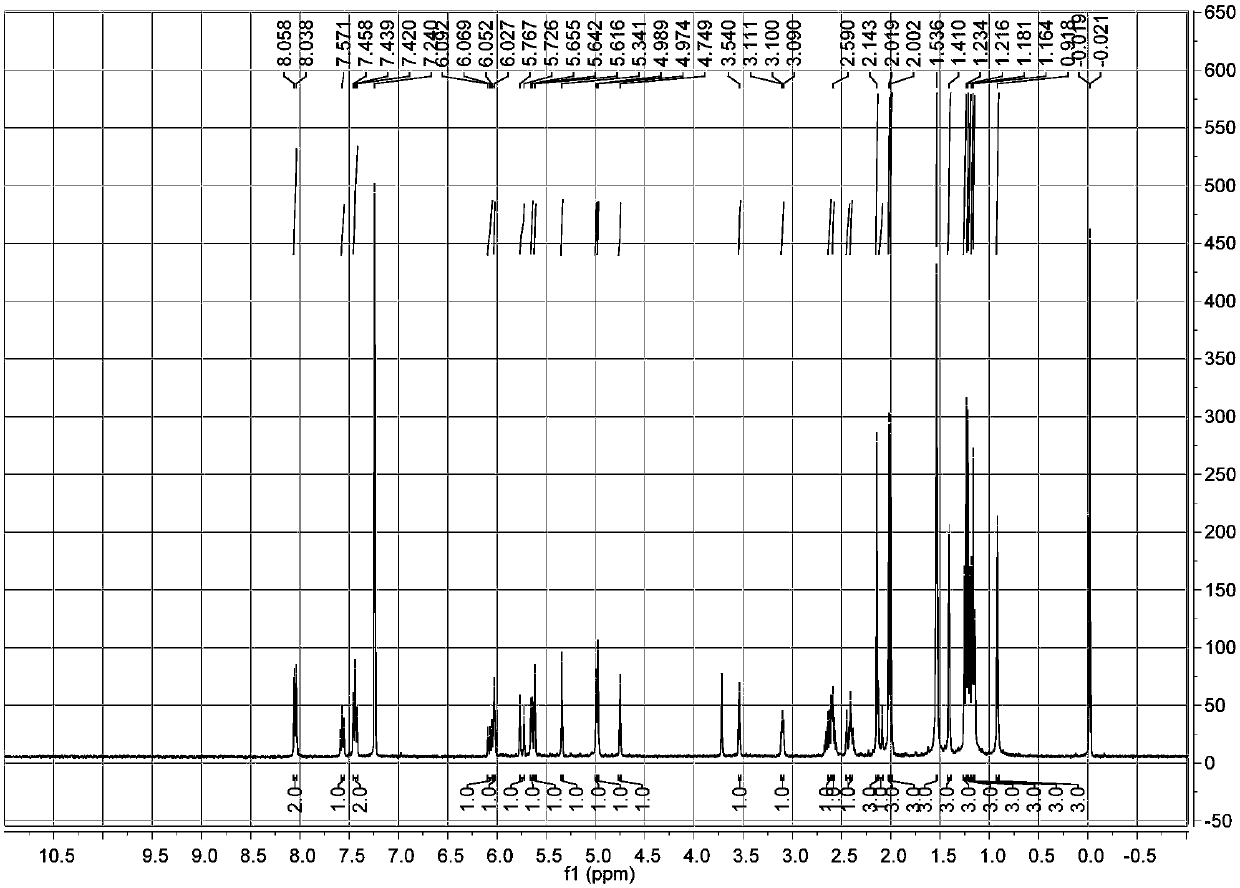
[0044] a. Get 10kg of the fruit of Euphorbia euphorbia, crush it and extract it by cold soaking at room temperature with 70L of dehydrated ethanol, and evaporate the solvent under reduced pressure to obtain the crude extract of Euphorbia ipsifolia;
[0045] b. Disperse the crude extract obtained in step a with ethanol, add cyclohexane for extraction, combine the ethanol layers and evaporate to dryness under reduced pressure to obtain the ethanol extract extract;
[0046] c. Separate the ethanol extract extract obtained in step b with a normal-phase silica gel column, carry out gradient elution with cyclohexane-ethyl acetate with a volume ratio of 10:1-0:1, and fractionate through silica gel thin-layer chromatography (TLC) analysis, the same fractions were combined to obtain 6 components (F1-F6); the component F4 was subjected to normal phase silica gel column separation, and the volume ratio was 100:0-0:100 with dichloromethane-acetone Gradient elution to obtain components F4A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com