Pyrazoline aminopeptidase N inhibitors as well as preparation method and application thereof
A technology of aminopeptidase and pyrazoline, which is applied in anti-inflammatory agents, antiviral agents, and pharmaceutical formulations, and can solve the problems of limited sources, poor selectivity, unfavorable scientific research, and large-scale production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
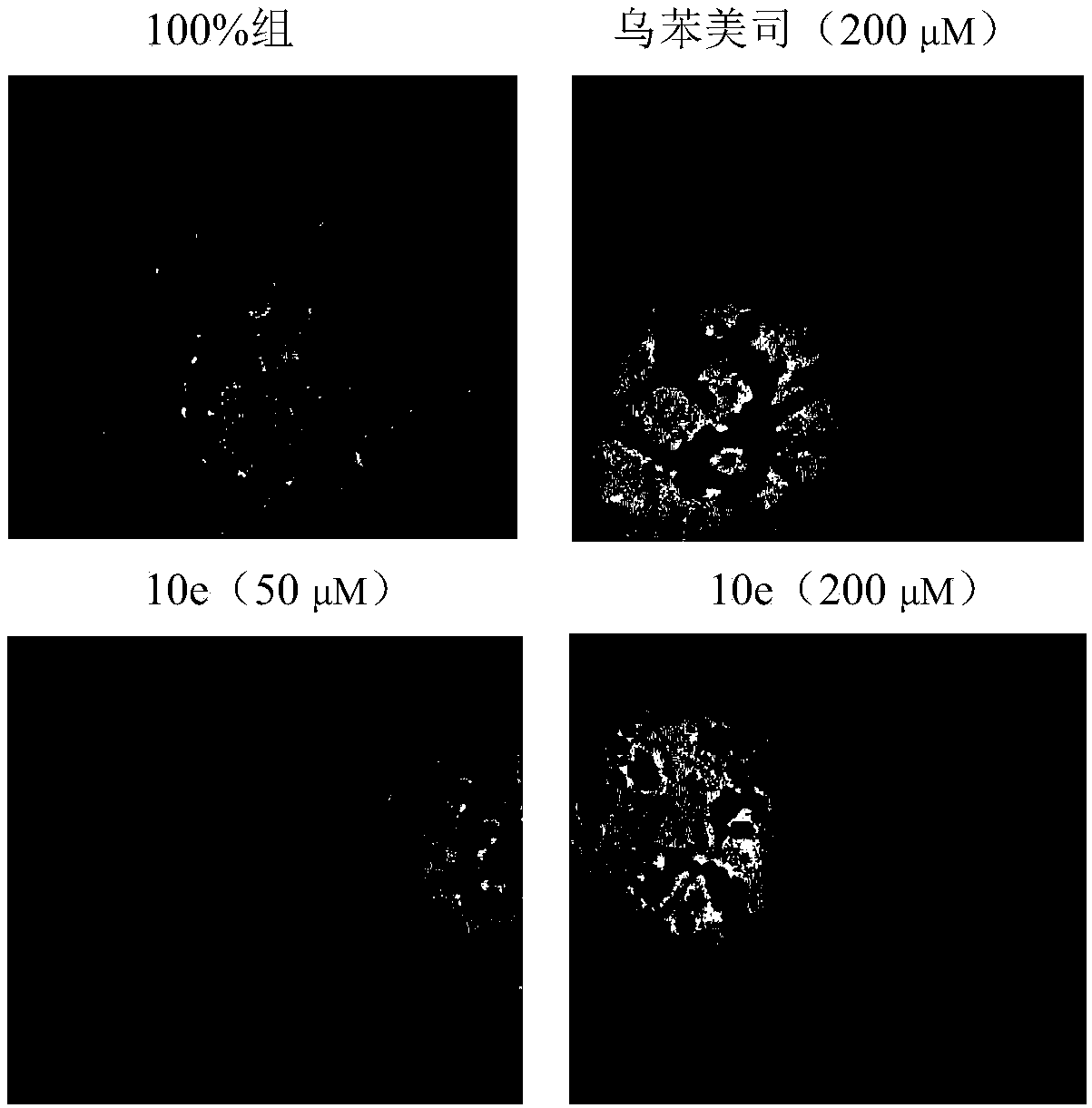
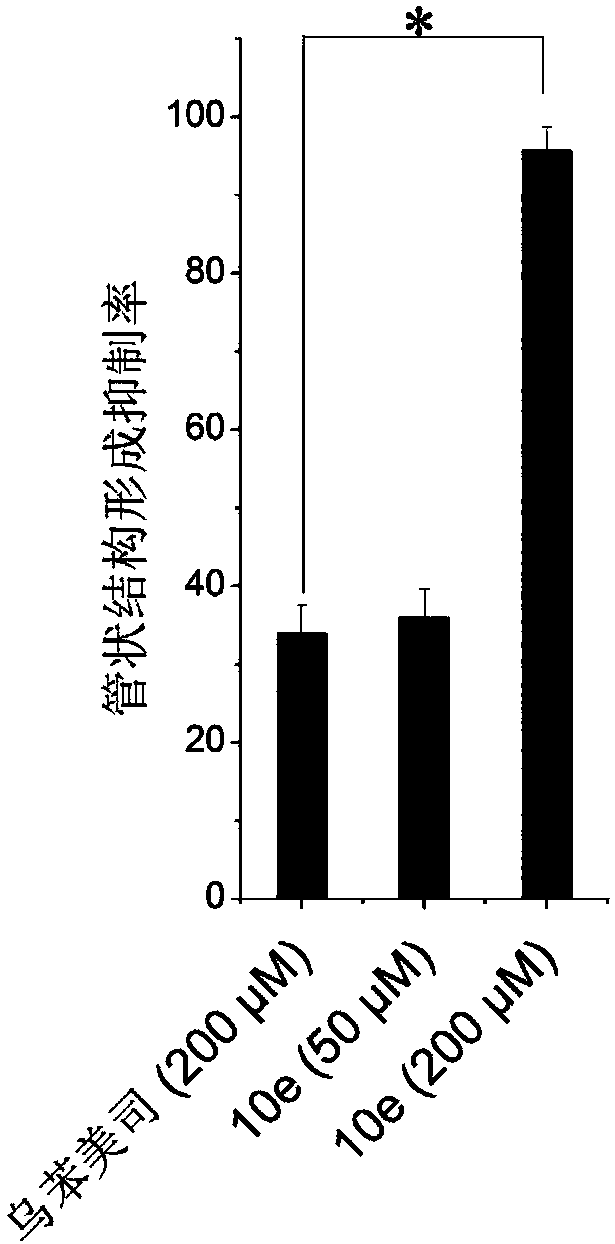
Image
Examples
Embodiment Construction
[0104] The terms and definitions used in this document have the following meanings:
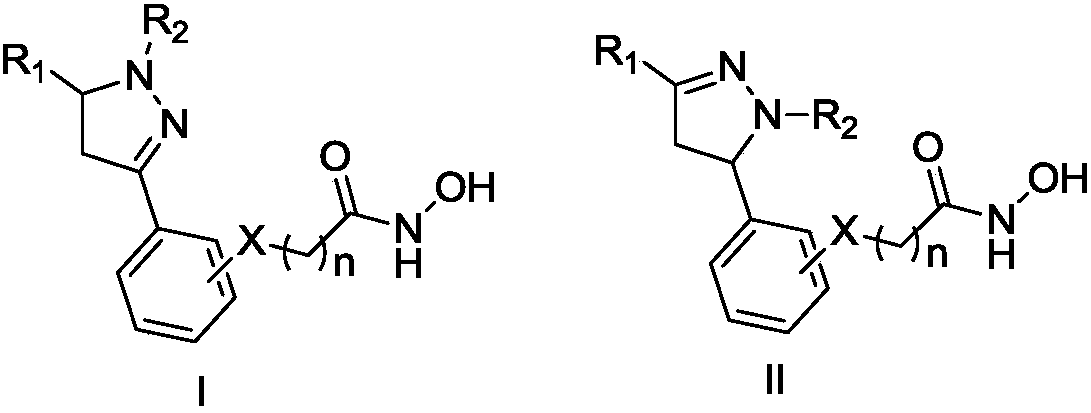
[0105] "Pharmaceutically acceptable salt" refers to a therapeutically effective and non-toxic salt form of a compound of formula (I) or (II). Many such salts are known in the art. A cationic salt formed on any acidic group such as carboxyl, or an anionic salt formed on any basic group such as amino. Many such salts are known in the art, such as cationic salts including alkali metal (such as sodium and potassium) and alkaline earth metal (such as magnesium and calcium) salts and organic salts (such as amine salts). Anionic salts can also be obtained by using the corresponding acids, such acids include mineral acids such as sulfuric acid, nitric acid, phosphoric acid, hydrochloric acid, etc.; or organic acids such as acetic acid, propionic acid, glycolic acid, 2-hydroxypropionic acid, 2-oxopropionic acid Acid, oxalic acid, malonic acid, succinic acid, maleic acid, fumaric acid, malic acid, ta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com