Compounds for treating rac-gtpase mediated disorder
A technology for compounds and compositions, which can be used in drug combinations, organic chemistry, bone diseases, etc., to solve problems such as decreased proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0100] The preparation of the compounds described in this application may involve the protection and deprotection of a wide variety of chemical groups. The need for protection and deprotection and selection of appropriate protecting groups can be readily determined by those skilled in the art. The chemistry of protecting groups can be found, for example, in T.W. Greene and P.G.M. Wuts, Protective Groups in Organic Synthesis, 3 rd Ed., Wiley & Sons, Inc., New York (1999), which is hereby incorporated by reference in its entirety.
[0101] The reaction can be monitored according to any suitable method known in the art. For example, product formation can be detected spectroscopically, e.g., nuclear magnetic resonance spectroscopy (e.g., 1 H or 13 C), infrared spectroscopy, spectrophotometry (eg UV-visible), mass spectroscopy or monitoring by chromatographic methods such as high performance liquid chromatography (HPLC), liquid chromatography-mass spectroscopy (LCMS) or thin la...
Embodiment 1
[0114] Example 1: Computer Simulation Screening of Rac Inhibitors
[0115] method
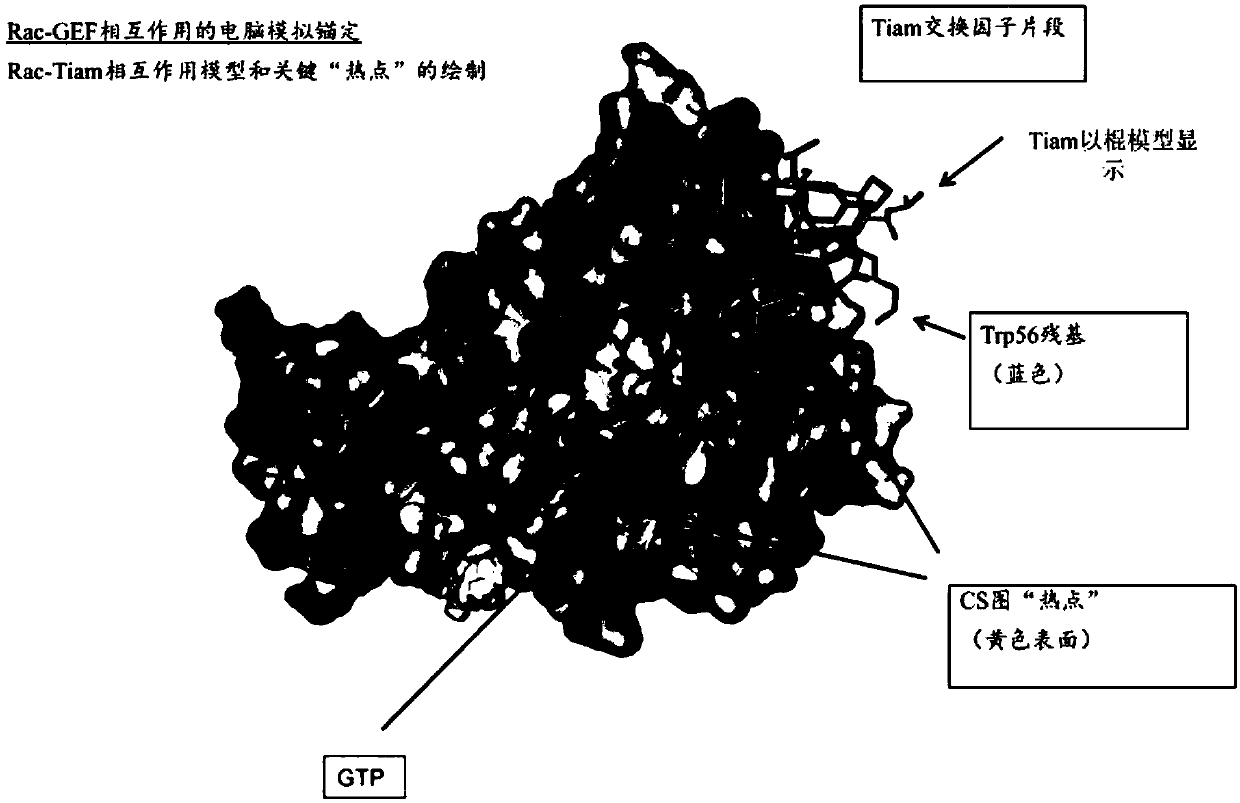
[0116] Rac-GEF Interaction In silico anchoring to the Rac2 crystal structure was required for viral screening for Rac inhibitors. In silico anchoring of the Rac-GEF interaction was performed to account for potential target sites on the Rac protein to which binding of small molecules would result in interference with the GEF-protein interaction. figure 1 Models of GEF TIAM with RAC (TIAM-Rac) are shown. Rac is shown in gray and TIAM is shown as the anchor model. The Trp56 residue of Tiam is highlighted in blue. Depicted are potential locations, "hot spots", for disrupting Rac-GEF interactions. Plotted "hot spots" are defined as target locations where Tiam and Rac have significant interactions, represented as yellow surfaces. GTP molecules are additionally present.
[0117]In silico screening screened 14 million compounds from the Evotex AG library. Specific filtering for drug-like propert...
Embodiment 2
[0119] Embodiment 2: screening guide compound
[0120] Dose-Dependent Proliferation Inhibition The 100 compounds selected from the initial in silico screen were assayed for proliferation inhibition by Cell Titer Glo in two leukemia cell lines, SEM and P12. Cells for proliferation assays were pelleted and resuspended. The cell suspension is then split and the compound to be tested is added at the desired concentration followed by plating. After the incubation period, Cell Titer Glo reagent (Promega CellTiter Aqueous phase non-radioactive cell proliferation assay), and incubated. Absorbance was then measured at 490 nm using a microplate reader. The effect of each compound on cell proliferation was analyzed.
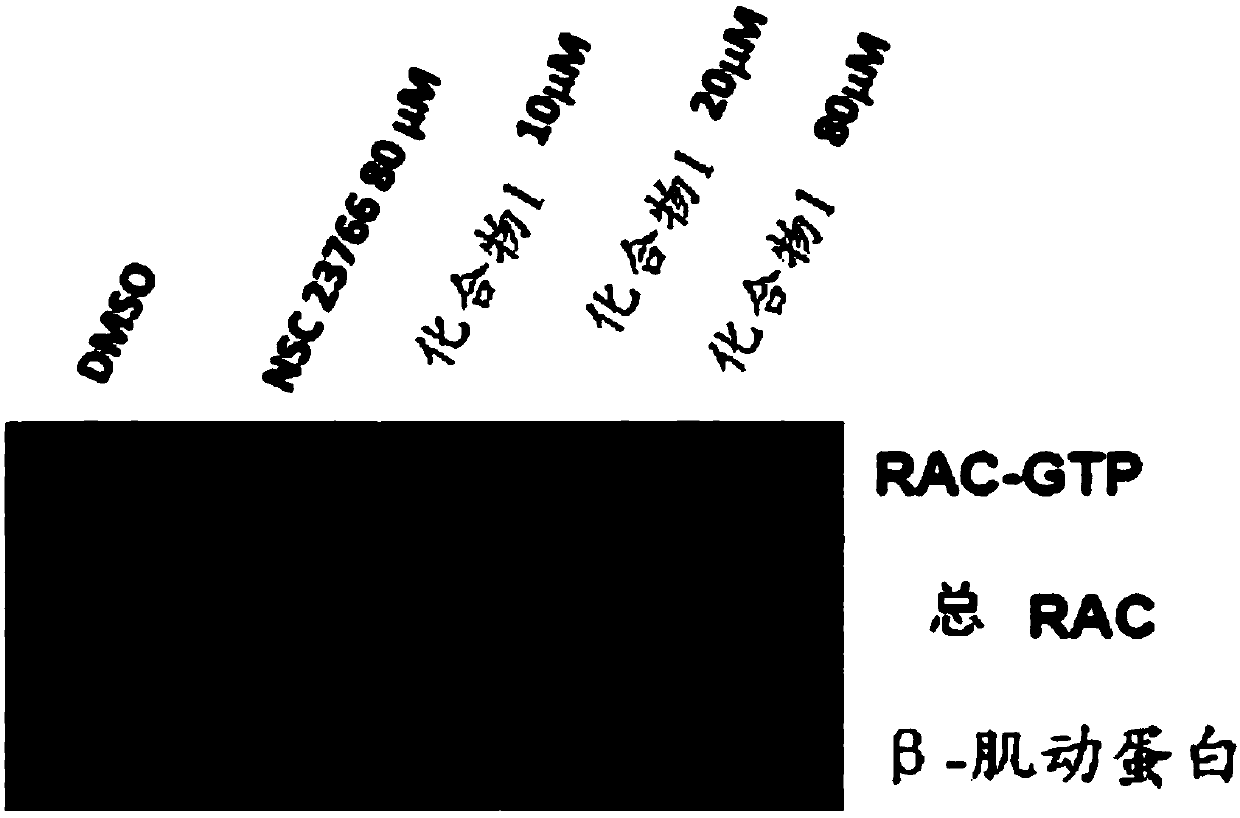
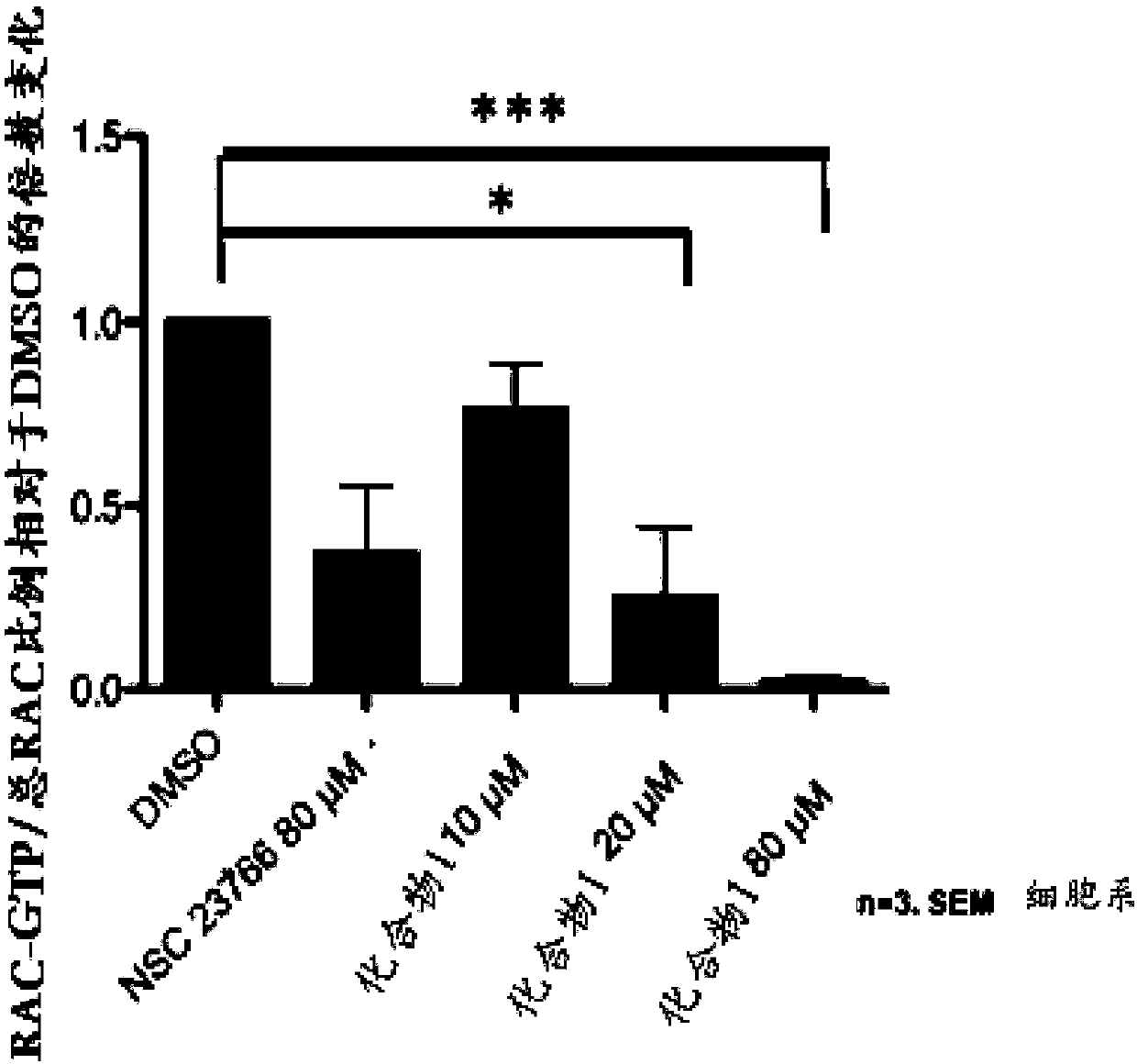
[0121] To determine which of these compounds should be pursued further, biochemical Pull-Down assays were performed to confirm specific inhibition of Rac activation indicated by disruption of the interaction between Rac and GTPase. The analysis initially requires pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com