Catalyst for preparation of pyromellitic dianhydride through durene oxidation
The technology of a pyromethylene and a catalyst is applied in the field of catalysts used for the oxidation of pyromethylene to produce homoanhydride, can solve the problems of high yield of homoanhydride, low yield of homoanhydride catalyst, etc., achieves improved activity and stability, improved Yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
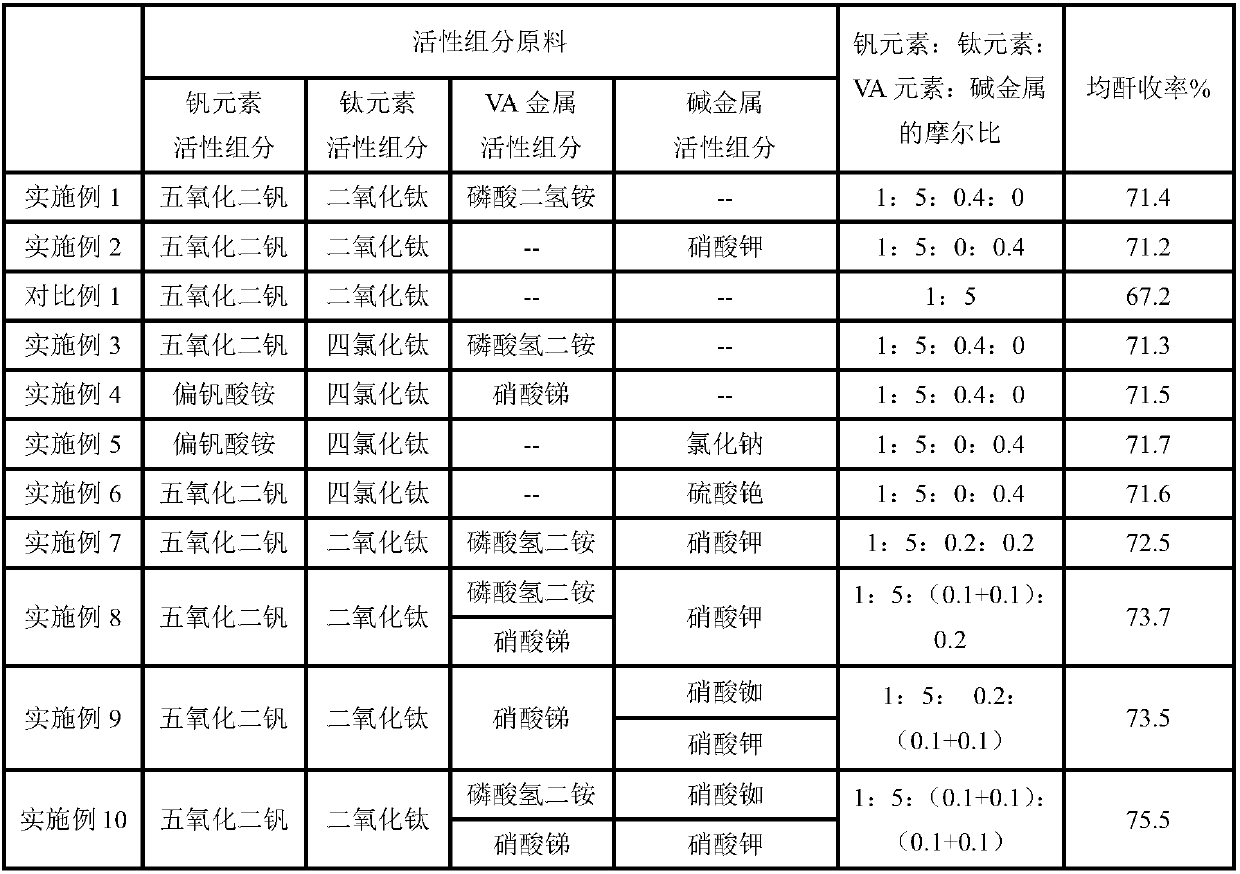
[0024] Weigh 88g of oxalic acid and 340ml of distilled water in a flask, stir and heat up to 80°C, and prepare an oxalic acid solution after the oxalic acid is completely dissolved. Add a portion of vanadium pentoxide to the prepared oxalic acid solution and continue stirring to obtain ammonium vanadyl oxalate solution. 5 parts TiO 2 , 0.4 parts of ammonium dihydrogen phosphate were added into the solution, and the catalyst precursor was obtained after continuing to stir evenly. After the catalyst precursor is filtered and dried, it is loaded into a spraying machine and evenly sprayed on the inert carrier α-alumina. The inert carrier sprayed with the catalyst precursor was calcined in a muffle furnace at 560 ° C, and the catalyst was obtained after natural cooling. Catalyst at a reaction temperature of 480°C and a space velocity of 5400h -1 Next, it was evaluated in a fixed-bed reactor, and the average anhydride yield was measured to be 71.4%. The evaluation results are sho...
Embodiment 2
[0026] Weigh 88g of oxalic acid and 340ml of distilled water in a flask, stir and heat up to 80°C, and prepare an oxalic acid solution after the oxalic acid is completely dissolved. Add a portion of vanadium pentoxide to the prepared oxalic acid solution and continue stirring to obtain ammonium vanadyl oxalate solution. 5 parts TiO 2 , 0.4 parts of potassium nitrate were added into the solution, and the catalyst precursor was obtained after continuing to stir evenly. After the catalyst precursor is filtered and dried, it is loaded into a spraying machine and evenly sprayed on the inert carrier α-alumina. The inert carrier sprayed with the catalyst precursor was calcined in a muffle furnace at 560 ° C, and the catalyst was obtained after natural cooling. Catalyst at a reaction temperature of 480°C and a space velocity of 5400h -1 Next, it was evaluated in a fixed-bed reactor, and the average anhydride yield was measured to be 71.2%. The evaluation results are shown in Table ...
Embodiment 3
[0031] Weigh 88g of oxalic acid and 340ml of distilled water in a flask, stir and heat up to 80°C, and prepare an oxalic acid solution after the oxalic acid is completely dissolved. Add a portion of vanadium pentoxide to the prepared oxalic acid solution and continue stirring to obtain ammonium vanadyl oxalate solution. Add 5 parts of titanium tetrachloride and 0.4 parts of diammonium hydrogen phosphate into the solution, and continue to stir evenly to obtain a catalyst precursor. After the catalyst precursor is filtered and dried, it is loaded into a spraying machine and evenly sprayed on the inert carrier α-alumina. The inert carrier sprayed with the catalyst precursor was calcined in a muffle furnace at 560 ° C, and the catalyst was obtained after natural cooling. Catalyst at a reaction temperature of 480°C and a space velocity of 5400h -1 Next, it was evaluated in a fixed bed reactor, and the average anhydride yield was measured to be 71.3%. The evaluation results are sh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com