A kind of preparation method of 2-bromo-5-chlorobenzaldehyde
A technology of chlorobenzaldehyde and bromosuccinimide, applied in the field of pharmaceutical synthesis, can solve problems such as low total yield, long reaction steps, complicated operation and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Put concentrated sulfuric acid (mass fraction 98%, 150mL) into the reaction kettle and stir to lower the temperature, control the temperature of the system to ≤10°C, slowly add compound A liquid (3-chlorobenzaldehyde, 0.5mol) dropwise, after the addition is complete, add an appropriate amount of catalyst I 2 (0.236-2.36mmol, the present embodiment is 30mg), add 3-chlorobenzaldehyde and catalyst I 2 When there is a relatively obvious exothermic phenomenon, it is necessary to control the reaction temperature of the system to ≤10°C;
[0036] System temperature control ≤ 15°C, add NBS (N-bromosuccinimide, 0.5mol) in batches for 5-10 times;
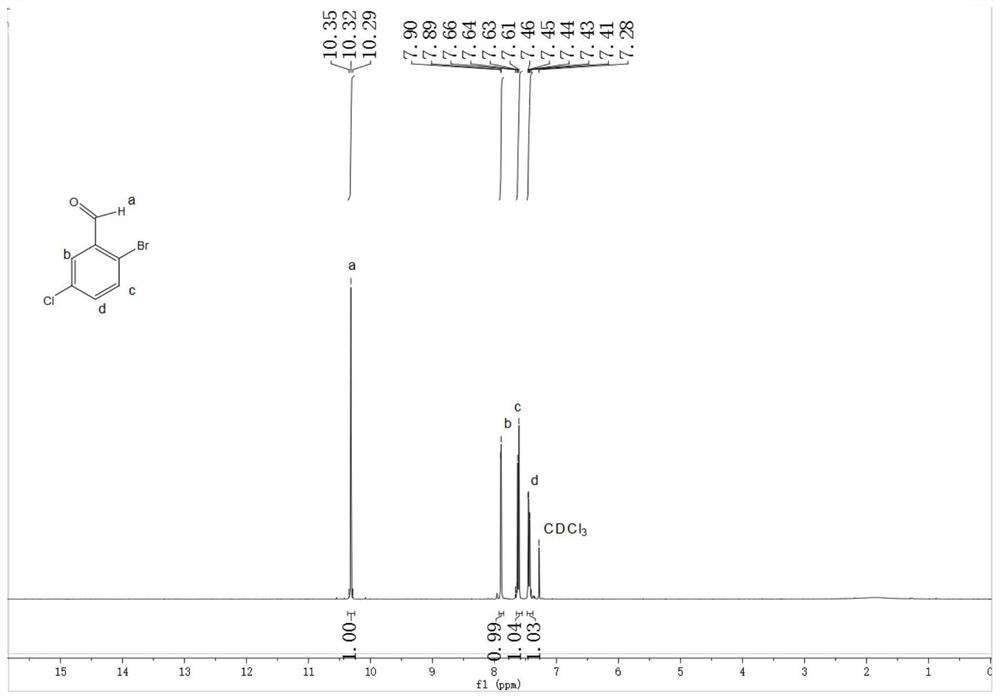
[0037] Insulate and react for 2 hours, slowly rise to 25°C, and then stir and react for 1 hour. GC detects that the content of 3-chlorobenzaldehyde in the raw material is ≤1%, and the reaction solution is post-processed and purified to obtain 98 g of needle-like off-white solid Compound B with a purity of 98.0%. Yield 90.1%.
Embodiment 2
[0039] Put concentrated sulfuric acid (mass fraction 98%, 100mL) into the reaction kettle and stir to lower the temperature, control the temperature of the system to ≤10°C, slowly add compound A liquid (3-chlorobenzaldehyde, 0.5mol) dropwise, after the addition is complete, add an appropriate amount of catalyst I 2 (0.236-2.36mmol, the present embodiment is 31.8mg), add 3-chlorobenzaldehyde and catalyst I 2 When there is a relatively obvious exothermic phenomenon, it is necessary to control the reaction temperature of the system to ≤10°C;
[0040] System temperature control ≤ 15°C, add NBS (N-bromosuccinimide, 0.5mol) in batches for 5-10 times;
[0041] Insulate and react for 2 hours, slowly raise to 25°C, and then stir for 1 hour. GC detects that the content of 3-chlorobenzaldehyde in the raw material is ≤1%, and the reaction solution is post-processed and purified to obtain 97.9 g of needle-like off-white solid compound B with a purity of 97.5%. , yield 90.0%.
Embodiment 3
[0043] Put concentrated sulfuric acid (98% mass fraction, 80mL) into the reaction kettle and stir to lower the temperature, control the temperature of the system to ≤10°C, slowly add compound A liquid (3-chlorobenzaldehyde, 0.5mol) dropwise, after the addition is complete, add an appropriate amount of catalyst I 2 (0.236-2.36mmol, the present embodiment is 63.5mg), add 3-chlorobenzaldehyde and catalyst I 2 When there is a relatively obvious exothermic phenomenon, it is necessary to control the reaction temperature of the system to ≤10°C;
[0044] System temperature control ≤ 15°C, add NBS (N-bromosuccinimide, 0.55mol) in batches for 5-10 times;
[0045] Insulate and react for 3 hours, slowly raise to 28°C, and then stir for 4 hours. GC detects that the content of 3-chlorobenzaldehyde in the raw material is ≤1%, and the reaction solution is post-processed and purified to obtain 98.5g of needle-like off-white solid Compound B with a purity of 98%. , yield 90.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com